| Identification | Back Directory | [Name]
4-Amino-2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione | [CAS]
19171-19-8 | [Synonyms]
IMiD 3
ActiMid
CC-4047
Pomalidomide
Pomalidomide-d4
Pomalidomide(CC-4047)
Pomalidomide(CC-4047,Actimid)
3-amino-N-(2,6-dioxo-3-piperidyl)phthalamide
3-Amino-N-(2,6-dioxo-3-piperidyl)phthalimide
4-Amino-2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione
4-AMino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline
4-Amino-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione
1H-Isoindole-1,3(2H)-dione,4-aMino-2-(2,6-dioxo-3-piperidinyl)- | [EINECS(EC#)]
805-902-5 | [Molecular Formula]
C13H11N3O4 | [MDL Number]
MFCD12756407 | [MOL File]
19171-19-8.mol | [Molecular Weight]
273.24 |
| Chemical Properties | Back Directory | [Melting point ]
318.5 - 320.5° | [Boiling point ]
582.9±45.0 °C(Predicted) | [density ]
1.570±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
DMSO: ≥14mg/mL | [form ]
powder | [pka]
10.75±0.40(Predicted) | [color ]
yellow | [Merck ]
14,135 | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. | [InChI]
InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) | [InChIKey]
UVSMNLNDYGZFPF-UHFFFAOYSA-N | [SMILES]
C1(=O)C2=C(C(N)=CC=C2)C(=O)N1C1CCC(=O)NC1=O |
| Hazard Information | Back Directory | [Chemical Properties]
Yellow Solid | [Usage]
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM | [Usage]
Pomalidomide is a second generation immunomodulator, TNF-α inhibitor, and thalidomide analog. An inhibitor of LPS-induced TNFαrelease. | [Usage]
Pomalidomide is a thalidomide derivative, a potent inhibitor of TNF-α production. It is an antiinflammatory and antitumor agent used in the treatment of multiple myeloma. | [Originator]
Celgene Corporation (United States) | [History]
Pomalidomide, a third-generation immunomodulatory agent (IMiD) following thalidomide and lenalidomide, was developed by Celgene in the United States. It represents a structural optimization and enhanced potency of thalidomide-like drugs. Its development stems from precise modifications to the thalidomide structure, particularly the addition of an amino group at the fourth carbon atom of the phthalimide ring. This improvement enhances its antitumor activity and immunomodulatory effects, aiming to overcome patient resistance to the first two generations of IMiD drugs. Pomalidomide exerts its potent anti-myeloma effect by binding to the intracellular Cerebrolysin (CRBN) protein and targeting the degradation of various tumor proteins. Its key clinical approval was based on the results of the MM-003 Phase III clinical trial, which showed that pomalidomide in combination with low-dose dexamethasone (Pom+LoDEX) significantly prolonged progression-free survival (PFS) in patients with relapsed and refractory multiple myeloma (RRMM). Based on this groundbreaking data, the U.S. FDA granted accelerated approval to pomalidomide on February 8, 2013, for the treatment of patients with RRMM who have received at least two prior lines of therapy, making it an important third- or fourth-line treatment option for refractory myeloma. Subsequently, its indication was expanded to include Kaposi's sarcoma in 2020. However, due to its structural similarity to thalidomide, pomalidomide remains subject to strict risk assessment and remission strategy (REMS) procedures worldwide to ensure its safety. | [Uses]
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM | [Uses]
Pomalidomide is a second generation immunomodulator, TNF-α inhibitor, and thalidomide analog. An inhibitor of LPS-induced TNFαrelease. | [Uses]
Pomalidomide is a thalidomide derivative, a potent inhibitor of TNF-α production. It is an antiinflammatory and antitumor agent used in the treatment of multiple myeloma. | [Definition]
ChEBI: An aromatic amine that is thalidomide substituted at position 4 on the isoindole ring system by an amino group. Used for the treatment of multiple myeloma in patients who failed to respond to previous therapies. | [Brand name]
Pomalyst | [reaction suitability]
reagent type: ligand | [Biochem/physiol Actions]
Pomalidomide is an effective fetal hemoglobin (HbF) inducer that downregulates the key γ-globin repressors, SRY-box transcription factor 6 (SOX6), and BAF chromatin remodeling complex subunit (BCL11A). | [Clinical Use]
Treatment of multiple myeloma | [Synthesis]
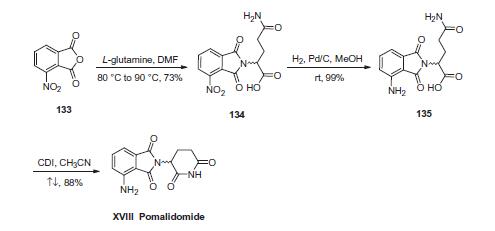
First, condensation of commercially available 3-nitrophthalic
anhydride (133) and L-glutamine in warm DMF gave nitrophthalimide
134. Although the authors from Celgene do not
explicitly describe the racemization of the stereocenter derived
from L-glutamine, scrambling of the stereocenter has been
reported during this step under neutral conditions at elevated
temperatures. Next, hydrogenative reduction of the nitro group
furnished the anilinophthalimide 135, and this was followed by
treatment with CDI in refluxing acetonitrile to secure the piperidone
dione and ultimately furnish pomalidomide (XVIII) as the
racemate in 87% overall yield from 134.
| [target]
TNF-α | [Drug interactions]
Potentially hazardous interactions with other drugs
Antidepressants: concentration increased by
fluvoxamine. | [Metabolism]
Mainly metabolised in the liver by the cytochrome P450
isoenzymes CYP1A2 and CYP3A4, with CYP2C19 and
CYP2D6 playing a minor role.
Following a single oral administration of
[14C]-pomalidomide (2 mg) to healthy subjects,
approximately 73% and 15% of the radioactive dose
was eliminated in urine and faeces, respectively, with
approximately 2% and 8% of the dosed radiocarbon
eliminated as pomalidomide in urine and faeces. | [storage]
Store at -20°C | [References]
1) Lopez-Girona?et al.?(2012),?Cereblon is direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide; Leukemia,?26?2326
2) Zhu?et al.?(2013),?Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma; Leukemia Lymphoma,?54?683
3) Donovan?et al.?(2018),?Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome; Elife,?7?e38430
4) Winter?et al.?(2015),?DRUG DEVELOPMENT. Phthalimide conjunction as a strategy for in vivo target protein degradation; Science,?348?1376
5) Lohbeck and Miller (2016),?Practical synthesis of a phthalimide-based Cereblon ligand to enable PROTAC development; Bioorg. Med. Chem. Lett.,?26?5260 |
| Questions And Answer | Back Directory | [Description]
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM in PBMCs. | [In vitro]
Pomalidomide inhibits lipopolysaccharide (LPS) stimulated TNF-alpha release in human PBMC and in human whole blood with IC50 values of 13 nM and 25 nM, respectively. Pomalidomide inhibits the growth of T regulatory cells which is stimulated by IL-2 with an IC50 of ~1 μM. Treatment with Pomalidomide (6.4 nM-10 μM) increases the production of IL-2 in human peripheral blood T cells, and is slightly more potent in the CD4+ subset than in the CD8+ subset. Pomalidomide is significantly more potent than CC-5013 at elevating IL-2, IL-5, and IL-10 levels, but only slightly more potent than CC-5013 at elevating IFN-γ levels.
Pomalidomide enhances SEE and Raji cells induced AP-1 transcriptional activity in Jurkat cells in a dose-dependent manner, with a maximal enhancement of 4-fold at 1 μM. Exposure of Raji cells to various concentrations of Pomalidomide (2.5-40 μg/mL) for 48 hours leads to a significant decrease in cell proliferation and DNA synthesis. There is a reduction of ~40% compared to vehicle-treated controls. | [In vivo]
Pomalidomide enhances the antitumor effect of rituximab against B-cell lymphomas in severe combined immunodeficient mice. Administration of Pomalidomide in combination with rituximab, gives the mice a median survival period of 74 days compared with 58 days of CC5013/rituximab treatment and 45 days of rituximab nonotherapy. The synergistic effect of Pomalidomide and rituximab can be completely abrogated by depletion of NK cells, supporting the proposal that NK cell expansion is one mechanism by which Pomalidomide may augment rituximab antitumor activity. |
|
|