| Identification | More | [Name]
Piroxicam | [CAS]
36322-90-4 | [Synonyms]
3,4-DIHYDRO-2-METHYL-4-OXO-N-2-PYRIDYL-2H-1,2-BENZOTHIAZINE-3-CARBOXAMIDE 1,1-DIOXIDE
4-HYDROXY-2-METHYL-3-(PYRID-2-YL-CARBAMOYL)-2H-1,2-BENZOTHIAZINE 1,1-DIOXIDE
4-HYDROXY-2-METHYL-3-(PYRID-2-YL-CARBAMOYL)-2H-1,2-BENZOTHIAZINE 1,2-DIOXIDE
4-HYDROXY-2-METHYL-N-2-PYRIDINYL-2H-1,2-BENZOTHIAZINE-3-CARBOXAMIDE
4-hydroxy-2-methyl-n-2-pyridinyl-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
AURORA KA-6753
PIROXICAM
PIROXICAMUM
2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide
2h-1,2-benzothiazine-3-carboxamide,4-hydroxy-2-methyl-n-2-pyridinyl-,1,1-diox
4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazin-3-caboxyamid-1,1-dioxid
4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazine-3-carboxamide-1,1-diox
4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide
Artroxicam
Baxo
Bruxicam
Caliment
chf1251
CP 16171
cp16171 | [EINECS(EC#)]
252-974-3 | [Molecular Formula]
C15H13N3O4S | [MDL Number]
MFCD00057317 | [Molecular Weight]
331.35 | [MOL File]
36322-90-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White to Pale Yellow Solid | [Melting point ]
198-200°C | [density ]
1.3664 (rough estimate) | [refractive index ]
1.6320 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Practically insoluble in water, soluble in methylene chloride, slightly soluble in anhydrous ethanol. It shows polymorphism (5.9). | [form ]
neat | [pka]
6.3 (2:1 dioxane-water) | [color ]
White to Light yellow | [biological source]
synthetic (organic) | [Water Solubility ]
Soluble in water, ethanol, chloroform, ethyl acetate. | [Usage]
Non-steroidal anti-inflammatory with long half-life. Cyclooxygenase inhibitor. Clinically useful NSAID | [λmax]
358nm(H2O)(lit.) | [Merck ]
14,7506 | [BCS Class]
2 | [InChIKey]
QYSPLQLAKJAUJT-UHFFFAOYSA-N | [CAS DataBase Reference]
36322-90-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Piroxicam(36322-90-4) | [EPA Substance Registry System]
2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide (36322-90-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,F | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 2811 | [WGK Germany ]
3 | [RTECS ]
DL0705000 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29349900 | [Toxicity]
LD50 orally in mice: 360 mg/kg (Wiseman) |
| Hazard Information | Back Directory | [Description]
Piroxieam is a non-steroidal anti-inflammatory drug, of
the oxieam class. A contact and photocontact sensitizer,
which induced contact dermatitis in a physieal
therapist. Piroxieam generally cross reacts with thiosalicylic
acid and also with thiomersal. Cross sensitivity
is not observed to tenoxicam. | [Chemical Properties]
Off-White to Pale Yellow Solid | [Originator]
Amida, Euphoric Pharmaceuticals | [Uses]
Non-steroidal anti-inflammatory with long half-life. Cyclooxygenase inhibitor. Clinically useful NSAID | [Uses]
Piroxicam is
used in inflammatory and degenerative diseases of the musculoskeletal system that are
accompanied by painful symptoms. It is used for rheumatic heart disease, nonspecific
infectious polyarthritis, gouty arthritis, rheumatoid arthritis, osteoarthritis, ankylosing
spondylitis, arthrosis, back pain, neuralgia, myalgia, and other diseases associated with
inflammation. | [Definition]
ChEBI: A monocarboxylic acid amide resulting from the formal condensation of the carboxy group of 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylic acid 1,1-dioxide with the exocyclic nitrogen of 2-aminopyridine. | [Indications]
Piroxicam
(Feldene) is indicated for the treatment of rheumatoid
arthritis and osteoarthritis. Piroxicam is a nonspecific
COX inhibitor that has a much higher affinity for
COX-1 than COX-2. This may account for the large
proportion (over 30%) of patients receiving long-term
therapy who have reported side effects.Adverse GI reactions
have been the most frequently reported side effect,
but edema, dizziness, headache, rash, and changes
in hematological parameters have also occurred in 1 to
6% of patients. Piroxicam can cause serious GI bleeding,
ulceration, and perforation, particularly in the elderly, if
the recommended dosage is exceeded or if aspirin is being
taken concurrently. | [Manufacturing Process]
189.6 g (3.51 mol) of sodium methoxide in 1.4 L of dry dimethylsulfoxide was stirred at room temperature (~ 25°C), while under a dry nitrogen atmosphere. To the stirred slurry, there were then added in one complete portion 300 g (1.17 moles) of methyl 3-oxo-1,2-benzoisothyazolin-2-acetate 1,1-dioxide (Chemische Berichte, vol. 30, p. 1267 (1897)) and flask containing the system was then immediately immersed in an ice-methanol bath. The resulting deep red solution was cooled to 30°C and the ice bath removed. The solution was then stirred under dry nitrogen at 30°C for 4 min, cooled quickly to 18°C and then immediately poured into 4.8 L of 3 N hydrochloric acid solution admixed with ice. The resulting slurry was stirred for 15 min, filtered, then washed with water to give 250 g of crude product. Recrystallization from a chloroform-ethanol mixture (1:1) in the presence of charcoal, then afforded a 61% yield of methyl 3,4-dihydro-4-oxo-2H-1,2benzothiazine-3-carboxylate 1,1-dioxide, melting point 173-174°C after two recrystallizations from isopropanol.
A 22 L round-bottomed flask charged with 800 g (3.13 moles) of methyl 3,4dihydro-4-oxo-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide, 3.2 l of water, 9.6 l of 95% ethanol, 673 ml of methyl iodide (1.53 kg, 10.87 moles) and 3.14 L of 1 N aqueous sodium hydroxide. The reaction mixture was then stirred for 30 min at room temperature, under nitrogen atmosphere and then solution was stored for 23 h. The slurry was then chilled at 0°C and filtered. After washing the filter cake twice with water, ethanol and then diethyl ether there were obtained 537 g of methyl 3,4-dihydro-2-methyl-4-oxo-2H-1,2benzothiazine-3-carboxylate 1,1-dioxide, melting point 165°-168°C after recrystallization from 1.25 L of acetonitrile.
In 3 L round-bottomed flask there were placed methyl 3,4-dihydro-2-methyl4-oxo-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide, 2-aminopyridin and dry xylene. Nitrogen gas was then bubbled into the suspension for 5 min, then the reaction mixture was heated to begin a period of slow distillation, with complete solution effected during the first 10 min of heating. After 5.5 h, the period of slow distillation was discontinued and reaction mixture was allowed to heat at reflux for approximately 16 h. After that the reaction mixture was cooled to room temperature and filtered. The solid material was crystallized from chloroform with methanol and againe from methanol and then there were obtained piroxicam, melting point 197°-200°C, dec. | [Brand name]
Feldene (Pfizer). | [Therapeutic Function]
Antiinflammatory, Analgesic | [General Description]
Piroxicam (Feldene) is the most widely used oxicam becauseof its once-daily dosing schedule. It is well absorbedafter oral administration and has a plasma half-life of 50hours, thus requiring a dose of only 20 to 30 mg oncedaily. It undergoes extensive hepatic metabolism, catalyzedby CYP2C9 to give 5-hydroxypiroxicam as its majormetabolite. Several piroxicam prodrugshave been synthesized via derivatization of the enol alcoholgroup (amipiroxicam, droxicam, and pivoxicam) to reducepiroxicam-induced GI irritation. | [Biological Activity]
Anti-inflammatory; highly selective inhibitor of COX-1 (ratio of IC 50 values for COX-2/COX-1 ~ 600). | [Biochem/physiol Actions]
Cyclooxygenase inhibitor. | [Clinical Use]
NSAID and analgesic | [Synthesis]
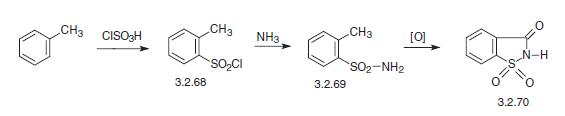
Piroxicam, 1,1-dioxid-4-hydroxy-2-methyl-N-2-pyradyl-2H-1,2-benzothiazine- 3-carboxamide (3.2.78), is synthesized from saccharin (3.2.70). Two methods for saccharin synthesis are described. It usually comes from toluene, which is sulfonated by chlorosulfonic acid, forming isomeric 4- and 2-toluenesulfonyl chlorides. The isomeric products are sepa�rated by freezing (chilling). The liquid part, 2-toluenesulfonyl chloride (3.2.68) is separated from the crystallized 4-toluenesulfochloride and reacted with ammonia, giving 2-toluenesul�fonylamide (3.2.69). Oxidation of the product with sodium permanganate or chromium (VI) oxide in sulfuric acid gives saccharin—o-sulfobenzoic acid imide (3.2.70).
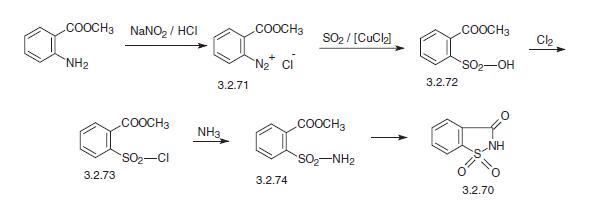
An alternative way for making saccharin is from methyl ester o-aminobenzoic (anthranylic acid). This undergoes diazotization using nitrous acid, and the resulting diazonium salt (3.2.71) is reacted with sulfur dioxide in the presence of copper dichloride, forming the methyl ester o-sulfobenzoic acid (3.2.72). Reaction of the resulting product with chlorine gives o-chlorosulfonylbenzoic acid methyl ester (3.2.73), which upon reaction with ammo�nium gives o-sulfonylamidobenzoic acid methyl ester (3.2.74). In the presence of hydro�gen chloride, the resulting product undergoes cyclization into saccharin (3.2.70).
| [Veterinary Drugs and Treatments]
In dogs, piroxicam may be beneficial in reducing the pain and inflammation
associated with degenerative joint disease, but there are
safer alternatives available. Its primary use is in dogs as adjunctive
treatment of bladder transitional cell carcinoma. It may also be of
benefit in squamous cell carcinomas, mammary adenocarcinoma,
and transmissible venereal tumor (TVT). There is some use of it
in cats for its anti-tumor effects, but it must be used with extreme
caution in this species. | [Drug interactions]
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration increased by ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity. | [Metabolism]
Piroxicam metabolism is mainly via cytochrome P450
CYP 2C9 in the liver by hydroxylation of the pyridyl ring
of the piroxicam side-chain, followed by conjugation with
glucuronic acid.
It is excreted mainly in the urine with smaller amounts in
the faeces. Enterohepatic recycling occurs. Less than 5% of
the dose is excreted unchanged in the urine and faeces. | [storage]
Store at 2-8°C |
|
|