| Identification | More | [Name]
ACETIC ACID | [CAS]
55896-93-0 | [Synonyms]
ACETATE ION CHROMATOGRAPHY STANDARD
ACETATE STANDARD
ACETIC ACID
ACETIC ACID, DILUTE R
ACETIC ACID, GLACIAL
ACETIC ACID R
ACIDUM ACETICUM
AKOS BBS-00003730
C2
CARBOXYLIC ACID C2
CHLOROSULFONYL ACETIC ACID ETHYL ESTER
CHLOROSULFONYL ACETIC ETHYL ESTER
ETHANOIC ACID
ETHANOLIC ACID
ETHYLIC ACID
FEMA 2006
GLACIAL ACETIC ACID
METHANECARBOXYLIC ACID
METHYLFORMIC ACID
RARECHEM AL BE 0544 | [EINECS(EC#)]
200-580-7 | [Molecular Formula]
C2H4O2 | [MDL Number]
MFCD00036152 | [Molecular Weight]
60.05 | [MOL File]
55896-93-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
16.2 °C(lit.)
| [Boiling point ]
117-118 °C(lit.)
| [density ]
1.049 g/mL at 25 °C(lit.)
| [vapor density ]
2.07 (vs air)
| [vapor pressure ]
11.4 mm Hg ( 20 °C)
| [refractive index ]
n20/D 1.371(lit.)
| [Fp ]
104 °F
| [storage temp. ]
Inert atmosphere,2-8°C | [form ]
Liquid | [pka]
4.76(at 25℃) | [color ]
Colorless | [Odor]
Characteristic vinegar, pungent; vinegar-like; sharp. | [Relative polarity]
0.648 | [Uses]
Colorless liquid prepared by the distillation of wood or the
oxidation of dilute ethyl alcohol. Acetic acid has a very pungent
smell, and the vapors are flammable. When obtained in full
strength (99 percent), it congeals as an ice-like solid at 16.7°C.
For this reason the term glacial is also used to describe this acid.
It is soluble in water, alcohol, ether, chloroform, and gelatin.
Acetic acid was used as a restrainer for the physical development
of calotypes, Niépceotypes, and collodion plates. Photographers
also used it to retard non-image reduction of these
processes by adding it to the silver nitrate solution. Acetic acid
is used as a stop bath and as a solvent for gelatin. | [CAS DataBase Reference]
55896-93-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2790 8/PG 2
| [WGK Germany ]
1
| [RTECS ]
AF1225000
| [HazardClass ]
8 | [PackingGroup ]
III |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
SULFO-ACETIC ACID ETHYL ESTER-->CHLOROSULFONYLACETYL CHLORIDE-->Ethanol-->Ethyl chloroacetate-->Ethyl Thioglycolate | [Preparation Products]
N-Isopropylacrylamide-->ETHYL 2-(2-FORMYL-4,5-DIMETHYL-1H-PYRROL-3-YL)ACETATE-->Sodium triacetoxyborohydride-->5-(TRIFLUOROMETHYL)-2-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->2-CYANO-5-BROMOPHENYLBORONIC ACID PINACOL ESTER-->2-(TRIFLUOROMETHYL)-6-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->5-Bromo-2-methoxypyrimidine-->3-METHOXY-2-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->2-METHOXY-6-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->4-CHLORO-2-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->2-CHLORO-6-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->Ethyl 2-Sulfamoylacetate-->2-BROMO-6-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->4-(TRIFLUOROMETHYL)-2-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->3-FLUORO-2-(5,5-DIMETHYL-1,3,2-DIOXABORINAN-2-YL)BENZONITRILE-->PYRIMIDINE-4,6-DICARBOYL DICHLORIDE-->(Diacetoxyiodo)benzene |
| Questions And Answer | Back Directory | [Background and overview]
An important chemical product. Colorless; irritating odor and sour corrosive liquids. More than 1000 BC, humans had begun to use acetate bacteria for wine fermentation to make vinegar. Vinegar has a acetic acid content of 2% to 12%. In 1911, Germany used acetaldehyde oxidation method to create the world's first synthetic acetic acid plant. In 1966 Monsanto, the United States developed the methanol low-pressure carbonylation process (built in 1970), which has become the main method of synthesizing acetic acid, accounting for more than 50% of the total output. In 1999, the world's acetic acid production was about 5 million tons. China began producing acetic acid in the 1950s, producing about 60,000 tons in the 1960s and increasing to 861,300 tons in 2001. The vast majority of acetic acid has been converted into derivatives for application. 40% to 50% has been used for the production of vinyl acetate in China and the United States. Both acetate ester and cellulose acetate accounts for 10% to 12%; solvent application accounts for 20% to 25%.
| [Uses]
- Used in the manufacture of vinyl acetate monomer (VAM), this accounts for one-third of acetic acid consumption.
 - As a food additive (condiment, ingredient) or as a preservative, it is found in Vinegar solutions, usually between 3-9% in concentration.
- In the production of various synthetic materials, acetic acid is a precursor and solvent for various glues and plastics such as polyvinyl acetate, cellulose acetate, nylon and dimethyl terephthalate.
- As an agent widely used in the manufacture of organic compounds which are constituent parts of food ingredients, various dye stuffs and perfumes, Rayon fibre, synthetic fibres and textiles, inks and dyes, soft drinks bottles, rubbers and plactics, and pesticides.
- In waste water treatment, acetic acid may be dosed to correct highly alkaline pH from where caustic soda has been dosed to the fluid stream.
- For testing blood in clinical laboratory
- For the treatment of outer ear infections from the growth of fungus and bacteria
| [Pharmacological effects]
This product has anti-bacterial and fungal infections. 5% solution has a bactericidal effect on Haemophilus and Pseudomonas. 0.5% ~ 2% solution has antiseptic effect on lavage wound sterilization; different concentrations of acetate can be used to treat various skin shallow fungal infections; the product has bactericidal efficacy. | [Synthetic route]
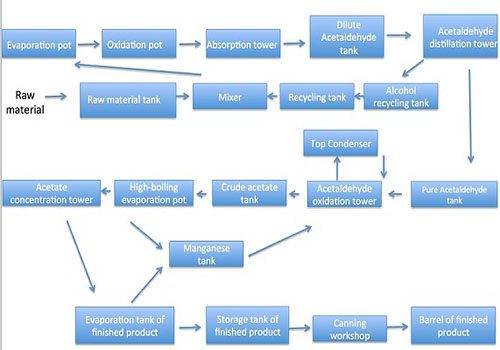
Alcohol oxidation: 95% of the raw alcohol and 76% of the alcohol recovered in the workshop are mixed in the mixing tank ratio into 84 ± 0.5% dilute alcohol, the alcohol ingredient is heated by evaporation pot into the oxidation furnace, and has reaction to generate acetaldehyde gas at 555 ± 5 ℃ high temperature and under the catalysis of electrolytic silver. The reaction gas mixture enters into the absorption tower after being condensed, and is diluted with about 8-10% of dilute acetaldehyde after being absorbed by water once. Acetaldehyde refining and alcohol recovery: dilute acetaldehyde is sent into the acetaldehyde distillation column for pressure distillation; control the top temperature at 45 ± 2 ℃ and pressure at 0.15Mpa; the tower gives pure acetaldehyde. Bottoms temperature should be controlled at 121 ± 3 ° C, the material is pressurized into the alcohol recovery column distillation with the tower temperature being controlled at 90 ± 5 ° C; the tower top finally produce about 76% alcohol to be used as the ingredient of the alcohol oxidation process; control the bottoms temperature at 110 ± 3 ℃, and the waste water is discharged through the tower kettle.
Acetaldehyde oxidation: Acetaldehyde enters into the oxidation tower through the action of the pressure pump; it has reaction with the compressed air at a temperature of 50 ~ 80 ℃, pressure 0.20 ~ 0.22Mpa and under a certain amount of manganese acetate to generate crude acetic acid. The crude acetic acid is discharged from the upper discharge port of the oxidation tower to the crude acetic acid storage tank; the unreacted acetaldehyde is condensed and separated from the top of the tower through the condenser, the liquid is refluxed to the bottom of the oxidation tower, and the exhaust gas is further absorbed into the rear of the oxidation tower by the bubbling absorber into the atmosphere.
Acetic acid refinement: The crude acetic acid is evaporated by the high-boiling pot to separate the manganese acetate from the heavy component, the temperature of the high-boiling evaporation pot is controlled at 120 ± 2 ℃, and the manganese acetate at the bottom of the high-boiling pot is discharged into the manganese circulation tank of the acetaldehyde oxidation process for recycling. The light fraction in the top enters into the concentration distillation column, and the bottoms temperature is controlled at 123 ± 3 ° C; acetic acid inside the tower is continuously and quantitatively discharged into the finished product evaporating pot and further distilled and condensed into acetate at 120 ± 2 ℃ into the finished product metering tank. After qualification, it is sent into the finished product pot. The temperature of the top of the tower is controlled at 100 ± 2 ℃. The dilute acid produced at the top of the tower enters into the measuring tank and is put into the dilute acid tank after measurement.
Production route includes BP Cativa process and Celaness AOPlus process.
BP Cativa process
BP is the world's largest provider of acetic acid, and 70% of the world's acetic acid production uses BP technology. BP introduced the Cativa technology patent in 1996. The Cativa process uses a new iridium-based catalyst system and uses a variety of new additives such as rhenium, ruthenium and osmium. The iridium catalyst system has higher activity than rhodium catalysts with few by-products and operates at lower water concentrations (less than 5%), which greatly improves traditional methanol carbonylation, cuts production costs by up to 30%, and reduces expansion costs by 50%. In addition, due to the decrease of water concentration, the CO utilization efficiency is increased and the steam consumption is reduced.
Celanese AOPlus process
Celanese is also one of the largest acetic acid producers in the world. In 1978, the Hurst-Celanese Company (now Celanese) commissioned a Monsanto acetic acid plant at Lake Clare in the U.S. state of Texas. In 1980, Celanese Corporation introduced the AOPlus method (acid optimization method) technology patents, greatly improving the Monsanto process. The AOPlus process increases the rhodium catalyst stability by adding high concentrations of inorganic iodine (mainly lithium iodide), and the water concentration in the reactor after the addition of lithium iodide and methyl iodide. | [Production]
Using methanol as a raw material, acetic acid is produced by low-pressure carbonylation. Methanol carbonylation is the main method for production of acetic acid.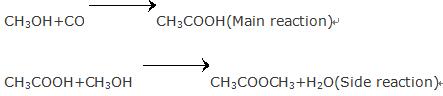
There are also many other synthetic ways industrially, which are listed as follows:
Acetaldehyde oxidation
Using acetaldehyde as a raw material, adopt liquid phase oxidation with air at 70° C. and 1 MPa, in order to prevent the occurrence of explosion caused by peroxide, cobalt acetate or manganese acetate could be used as a peroxide decomposer under an exothermic reaction. Cool and control the reaction temperature at 70 °C, acetic acid can be obtained by the following concentration and distillation.
Celanese
With butane as raw material, manganese acetate is used as a catalyst at 150-250°C and 6MPa to oxidize with air to obtain acetic acid.
BP Chemical
Using oils containing more than 40% C4-C8 aliphatic hydrocarbons as a raw material, adopt the liquid phase oxidation in the presence of manganese acetate or cobalt acetate at a temperature of 160-170° C. and 4.0 MPa, and yields of acetic acid, formic acid, propionic acid, and butyric acid are generated. Operation of distillation and refining are followed to get acetic acid.
It can be prepared using ethylene as raw material, palladium as catalyst and vanadium as co-catalyst under oxidation reaction. | [Indications]
This product has anti-bacterial and fungal infections. 2% to 5% solution has a bactericidal effect on Haemophilus and Pseudomonas. It also has effect on Candida, Aspergillus and Trichomonas. It also has spermicidal effect. Various concentrations can be used for the treatment of various skins shallow bacterial or fungal infections, but also for vaginal trichomoniasis, burn wound infection, prevention of flu or flu and contraception. | [Specification]
Acetic acid solution: 0.1% ~ 5% (concentration required). | [Dosage]
- Onychomycosis: Cotton ball immersed in 30% glacial acetic acid solution is placed on the sick arm, once daily and once for 10-15 minutes until disease A is removed and the treatment continues for 2 weeks.
- Hand, foot and ringworm: Submerge feet with 10% glacial acetic acid solution once daily and once for 10 minutes for 10 consecutive days. If not cured, repeat once every other week.
- Pityriasis: coated with 5% glacial acetic acid solution 2 times a day.
- Body ringworm: rub with 5% ~ 10% glacial acetic acid solution, 2 times a day.
- Corn and wart: apply the affected area with 30% glacial acetic acid once daily.
- Lavage the wound: with 0.5% ~ 2% solution.
| [Adverse reactions]
Can cause contact dermatitis. 30% solution of onychomycosis can cause chemical paronychia. There are also tingling and burning sensations. | [Precautions]
Avoid contact with eyes; all kinds of shallow skin fungal infections can be treated with different concentrations of this product.
- treatment of onychomycosis: after cleaning the lesion, use blunt knife to skive the onychomycosis, be careful not to contact a ditch; can coat a layer of vaseline for protection in nearby skin;
- facial ringworm disease should be not treated with this product;
- high concentration of acetic acid has a corrosive effect, avoid using it for the treatment of other ringworm except onychomycosis;
- treatment of corn; clean the lesions first and immerse in hot water for 15 to 30 minutes; use vaseline to protect nearby normal skin before applying medicine.
| [Taboo]
Allergic patients and patients of otitis media perforation should be disabled. | [Solidifying fixatives]
Acetic acid is a colorless and transparent liquid, being highly irritating. It is condensed into ice in cold condition, thus being also known as glacial acetic acid. Acetic acid can be formulated into solutions of various ratios using water and alcohol; the concentration ranges from 0.2 to 5%; it is often used together with other fixatives. It has strong penetration capability with single use causing protoplasm expansion, thus often being used in combination with alcohol, formaldehyde. Acetic acid is an excellent fixative of chromosomes, so the fixing solutions of chromosomes almost all contain acetic acid. Features: strong penetration capability; can cause tissue expansion. |
| Hazard Information | Back Directory | [Brand name]
Vosol (Carter-Wallace). | [Biotechnological Production]
Acetic acid is produced for beverage, food, and feed applications almost entirely
using the traditional vinegar process . First, ethanol is produced by fermentation
with Saccharomyces cerevisiae in the absence of oxygen. Then, acetic acid
is generated from ethanol by acetic acid bacteria, such as Acetobacter aceti,
Acetobacter pasteurianus, or Gluconacetobacter europaeus, under aerobic conditions. Different substrates, such as malt, fruits, and sugarcane, are used
for vinegar production . Today, processes with two stages (e.g. two-tank cycle
fermentation or two-stage submerged fermentation) are generally employed on an
industrial scale. In a first step, biomass is produced in parallel to the acetic acid
production. In the second part of the process, mainly acidification takes place.
Acetic acid concentrations up to 200 g.L-1 can be achieved .
The vinegar process has been well studied over many decades . However,
there are still attempts to enhance vinegar production, especially regarding productivity and cost minimization through alternative substrates, new
process concepts (e.g. immobilized cells or mixed cultures of yeasts and
acetic acid bacteria, and optimized acetic acid bacteria.
Acetic acid can be produced under anaerobic conditions by some microorganisms
such as Clostridium thermoaceticum . In free-cell batch fermentations,
acetate concentrations of 50 g.L-1 were reached in less than 192 h. Acetic
acid concentrations of 83–100 g.L-1, a yield of 0.74–0.80 g acetic acid per gram
glucose, and a productivity of 0.60–0.85 g.L-1.h-1 were observed under optimized
conditions in a cell-recovered fed-batch process with pH-control using
glucose as substrate. | [Health Hazard]
Recommended Personal Protective Equipment: Protective clothing should be worn when skin contact can occur. Respiratory protection is necessary when exposed to vapor. Complete eye protection is recommended; Symptoms Following Exposure: Breathing of vapors causes coughing, chest pains, and irritation of the nose and throat; may cause nausea and vomiting. Contact with skin and eyes causes burns; General Treatment for Exposure: INHALATION: Move the victim immediately to fresh air. If breathing becomes difficult, give oxygen and get medical attention immediately. INGESTION: If the victim is conscious, have him drink water or milk. Do not induce vomiting. SKIN OR EYE CONTACT: Flush immediately with lots of clean running water; wash eyes for at least 15 min. and get medical attention as quickly as possible; remove contaminated clothing; Toxicity by Inhalation (Threshold Limit Value): 10 ppm; Short-Term Exposure Limits: 40 ppm for 5 min.; Toxicity by Ingestion: LD50 0.5 to 5.0 g/kg (rat); Late Toxicity: No data; Vapor (Gas) Irritant Characteristics: Vapors cause moderate irritation such that workers will find high concentrations very unpleasant. Effects are temporary; Liquid or Solid Irritant Characteristics: This is a fairly severe skin irritant; may cause pain and secondary burns after a few minutes of contact; Odor Threshold: 1.0 ppm. | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: Corrosive, particularly when diluted. Attacks most common metals including most stainless steels. Excellent solvent for many synthetic resins or rubber; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Dilute with water, rinse with sodium bicarbonate solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
|