| Identification | More | [Name]
Naftifine | [CAS]
65472-88-0 | [Synonyms]
(e)-n-methyl-n-(1-naphthyl methyl)-3-phenyl-2-propen-1-amine
NAFTIFINE
n-trans-cinnamyl-n-methyl-(1-naphthylmethyl)amine
naftifin
METHYL-NAPHTHALEN-1-YLMETHYL-((E)-3-PHENYL-ALLYL)-AMINE
Naftifinebase
N-TRANS-CINNAMYL-N-METHYL-(1-NAPPHTHYLMETHYL)AMINE HYDROCHLORIDE
(E)-N-Methyl-N-(3-phenyl-2-propenyl)-1-naphthalenemethanamine
AW-105-843
Exoderil
Naftifungin
Naftin
SN-105-843
N-[(E)-3-Phenyl-2-propenyl]-N-methylnaphthalene-1-methanamine
N-Methyl-N-[(E)-3-phenyl-2-propenyl]-1-naphthalenemethanamine | [Molecular Formula]
C21H21N | [MDL Number]
MFCD00865622 | [Molecular Weight]
287.4 | [MOL File]
65472-88-0.mol |
| Hazard Information | Back Directory | [Originator]
Naftifine,Sandoz (Novartis) | [Uses]
(E)-Naftifine is an intermediate in synthesizing Naftifine N-Oxide (N213110), which is an impurity or metabolite of Naftifine Hydrochloride (N213100), an allylamine antifungal agent. | [Uses]
Naftifine (Naftin) is a synthetic allylamine derivative topical antifungal agent that works by blocking squalene 2,3-epoxidase, resulting in increased cell membrane permeability and cell death. It is structurally and pharmacologically related to terbinafine. It also has some antiinflammatory properties that may be due to its ability to alter chemotaxis by polymorphonucleocytes. It is most effective against dermatophytes, moderately active against molds, and less active against yeasts, including C. albicans.
| [Uses]
Naftifine is only permitted to be used externally and only superficially as a drug with a
broad spectrum of action against dermatophytes and candida infections. According to the
initial data, it exceeds the activity of econazole. Moreover, it does not have a locally irri�tating effect. It is believed that the fungicide activity of this drug is based on its ability to
inhibit the fungal enzyme squalene epoxidase, thus lowering the concentration of ergos�terol. The corresponding enzyme in mammals is inhibited significantly less. Synonyms of
this drug are exoderil, naftin, and others. | [Definition]
ChEBI: A tertiary amine in which the nitrogen is substituted by methyl, alpha-naphthylmethyl, and (1E)-cinnamyl groups. It is used (usually as its hydrochloride salt) for the treatment of fungal skin infections. | [Indications]
Naftifine (Naftin) is a synthetic allylamine derivative topical antifungal agent
that works by blocking squalene 2,3-epoxidase, resulting in increased cell
membrane permeability and cell death. It is structurally and pharmacologically
related to terbinafine. It also has some antiinflammatory properties that
may be due to its ability to alter chemotaxis by polymorphonucleocytes. It is
most effective against dermatophytes, moderately active against molds, and
less active against yeasts, including C. albicans. | [Manufacturing Process]
To a mixture of 1.42 g of methyl-(1-naphthylmethyl)amine hydrochloride,
2.89 g of sodium carbonate and 10 ml of dimethylformamide is added, at
room temperature, 1.25 g of cinnamyl chloride, dropwise. After 18 hours
stirring, at room temperature, the mixture is filtered and the filtrate is
evaporated in vacuo. The residue is dissolved in toluene and, after drying over
sodium sulphate, evaporated to obtain the trans-N-(cinnamylmethyl)-N�methyl-(1-naphthylmethyl)amine compound, boiling point 162-167°C/0.015
Torr.
The free base may be converted, with isopropanolic hydrogen chloride
solution, into the hydrochloride form, melting point 177°C (from propanol). | [Brand name]
Naftin (Merz). | [Therapeutic Function]
Antifungal | [Synthesis Reference(s)]
Journal of Medicinal Chemistry, 29, p. 112, 1986 DOI: 10.1021/jm00151a019
Tetrahedron Letters, 25, p. 2535, 1984 DOI: 10.1016/S0040-4039(01)81224-7 | [Pharmaceutical Applications]
A topical antifungal used as a 1% cream for the treatment
of dermatophytoses, including tinea pedis, tinea corporis and
tinea cruris. | [Clinical Use]
Naftifine was the first allyl amine to be discovered and marketed. It is subject to extensive first-pass metabolism to be orally active and, consequently, is only available in topical preparations. The widest use of naftifine is against various tinea infections of the skin. | [Synthesis]
Naftifine, (E)-N-methyl-N-(3-phenyl-2-propenyl)-1-naphthalinmethanamine
(35.3.1), is synthesized by alkylating N-methyl-(1-naphthylmethyl)-amine with cinnamyl
chloride in the presence of sodium carbonate.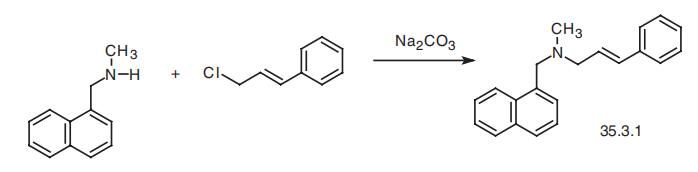
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|