| Identification | More | [Name]
Cefadroxil | [CAS]
66592-87-8 | [Synonyms]
(6R,7R)-7-[(R)-2-AMINO-2-(4-HYDROXY-PHENYL)-ACETYLAMINO]-3-METHYL-8-OXO-5-THIA-1-AZA-BICYCLO[4.2.0]OCT-2-ENE-2-CARBOXYLIC ACID
CEFADROXIL
CEFADROXIL MONOHYDRATE
CEFAMOX
P-HYDROXYCEPHALEXINE
monohydrate,(6r-(6-alpha,7-beta(r*)))-l)acetyl)amino)-3-methyl-8-oxo
Cefradroxil
(6R,7R)-7-[[(2R)-Amino-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid Monohydrate
Baxan
Cefa-Drops
Duracef
MJF-11567-3
p-Hydroxycephalexine Monohydrate
5-Thia-1-azabicyclo4.2.0oct-2-ene-2-carboxylic acid, 7-(2R)-amino(4-hydroxyphenyl)acetylamino-3-methyl-8-oxo-, monohydrate, (6R,7R)-
7-[[2-Amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
AMOXYCILLIN
CEFADDROXIL
(6R,7R)-7-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid hydrate
5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-((amino(4-hydroxyphenyl)acetyl) amino)-3-methyl-8-oxo-, monohydrate, (6R-(6-alpha,7-beta(R*)))
Cefadroxil hydrate | [EINECS(EC#)]
629-747-6 | [Molecular Formula]
C16H17N3O5S | [MDL Number]
MFCD00865091 | [Molecular Weight]
363.39 | [MOL File]
66592-87-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
197 °C | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
Slightly soluble in water, very slightly soluble in ethanol (96 per cen | [form ]
neat | [pka]
pKa 1.38(H2O t=20±2 N2atmosphere) (Uncertain); 7.35(H2O t=20±2 N2atmosphere) (Uncertain);10.10(H2O t=20±2 N2atmosphere) (Uncertain) | [color ]
White to Off-White | [Usage]
Semi-synthetic cephalosporin antibiotic. Antibacterial | [BCS Class]
3 | [InChIKey]
NBFNMSULHIODTC-CYJZLJNKSA-N | [SMILES]
N12C([C@@H](NC(=O)[C@H](N)C3=CC=C(O)C=C3)[C@@]1([H])SCC(C)=C2C(=O)O)=O.O |&1:2,6,15,r| | [CAS DataBase Reference]
66592-87-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
2
| [RTECS ]
XI0337000
| [HS Code ]
2941906000 |
| Hazard Information | Back Directory | [Description]
Cefadroxil has an amoxicillin-like side chain at C-7 and is orally active. There are some indications that
cefadroxil has some immunostimulant properties mediated through T-cell activation and that this is of
material assistance to patients in fighting infections. The prolonged biological half-life of cefadroxil allows
once-a-day dosage. | [Chemical Properties]
White Crystalline Solid | [Uses]
Cefadroxil Monohydrate is a semi-synthetic cephalosporin antibiotic. Antibacterial. | [Uses]
cephalosporin antibacterial | [Uses]
Semi-synthetic cephalosporin antibiotic. Antibacterial | [Definition]
ChEBI: The hydrate that is the monohydrate of the cephalosporin cefadroxil. | [Brand name]
Duricef (Bristol-Myers Squibb);
Ultracef (Bristol Labs). | [Antimicrobial activity]
Resembles closely that of cefalexin | [Application]
Cefadroxil was found by Bristol-Myers Co. in 1976. A hydroxyl group was attached to the benzene ring of cephalexin. Like amoxicillin and ampicillin, cefadroxil shows almost the same antibacterial activity spectrum as cephalexin and superior oral absorption. Its in vivo activity is four to six times greater than that of cephalexin, and its half-life in serum is twice as long. | [General Description]
Cefadroxil is a semisynthetic first-generation β-lactam cephalosporin antibiotic derived from cefalexin with antibacterial activity. It is used to treat urinary tract infections, skin and skin structure infections, pharyngitis, and tonsillitis caused by susceptible bacteria. | [Pharmacokinetics]
Oral absorption: >90%
Cmax 250 mg oral: c. 9 mg/L after 1.2 h
500 mg oral: c. 18 mg/L after 1.2 h
Plasma half-life: 1–1.5 h
Plasma protein binding : 20%
Absorption is little affected by administration with food.
Distribution is similar to that of cefalexin. It is eliminated
unchanged by glomerular filtration and tubular secretion;
90% of the dose appears in the urine over 24 h, most in the
first 6 h, producing concentrations exceeding 500 mg/L. | [Pharmacology]
Cefadroxil has a broad spectrum of antimicrobial action; it is active with respect to Gram�positive and Gram-negative microorganisms. Like all of the other drugs described above,
it acts as a bactericide by disrupting the process of restoring the membranes of bacteria.
Synonyms of this drug are bidocef, cefadril, duracef, ultracef, and others. | [Clinical Use]
Cefadroxil (Duricef) is an orally active semisyntheticderivative of 7-ADCA, in which the 7-acyl group is the Dhydroxylphenylglycylmoiety. This compound is absorbedwell after oral administration to give plasma levels that reach75% to 80% of those of an equal dose of its close structuralanalog cephalexin. The main advantage claimed for cefadroxilis its somewhat prolonged duration of action, whichpermits once-a-day dosing. The prolonged duration of actionof this compound is related to relatively slow urinary excretionof the drug compared with other cephalosporins, butthe basis for this remains to be explained completely. Theantibacterial spectrum of action and therapeutic indications ofcefadroxil are very similar to those of cephalexin and cephradine.The D-p-hydroxyphenylglycyl isomer is much moreactive than the L-isomer. | [Clinical Use]
It has been used for various community-acquired infections
for which oral cephalosporins are appropriate. | [Side effects]
Side effects described are those common to oral cephalosporins. | [Synthesis]
Cefadroxil, [6R-[6|á,7|?(R)]]-3-methyl-8-oxo-7-[[amino(4-hydroxyphenyl)
acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.14), is an ana�log of cephalexin and differs only in the presence of a hydroxyl group in the fourth posi�tion of the phenyl ring of phenylglycine, and is synthesized by a scheme analogous to the
scheme of cephradin synthesis.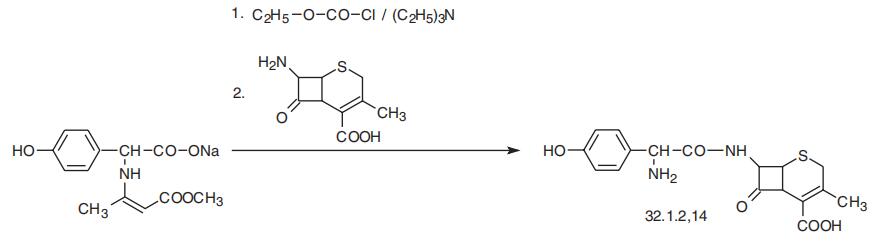
| [Veterinary Drugs and Treatments]
Cefadroxil is approved for oral therapy in treating susceptible infections
of the skin, soft tissue, and genitourinary tract in dogs and cats.
The veterinary oral tablets have been discontinued (in the USA), but
human-labeled oral capsules and tablets are still available. | [in vitro]
the inhibitory activity of this compound was similar to that of cephalexin and cephradine when tested against 602 clinical isolates on mueller-hinton medium. in the oral treatment of experimental infections of mice, cefadroxil was more effective than cephalexin against streptococcus pyogenes, and comparably effective against streptococcus pneumoniae, staphylococcus aureus, and several gram-negative species [1]. | [in vivo]
in mice, oral administration of cefadroxil at doses ranging from 25 to 100 mg/kg attained peak concentrations in the blood. higher peak levels were noted with cefadroxil than with cephalexin at a dose of 200 mg/kg [1]. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be
enhanced. | [Metabolism]
More than 90% of a dose of cefadroxil may be excreted
unchanged in the urine within 24 hours by glomerular
filtration and tubular secretion. | [References]
[1] buck r e, price k e. cefadroxil, a new broad-spectrum cephalosporin[j]. antimicrobial agents and chemotherapy, 1977, 11(2): 324-330.
[2] gerber m a, randolph m f, chanatry j, et al. once daily therapy for streptococcal pharyngitis with cefadroxil[j]. the journal of pediatrics, 1986, 109(3): 531-537. |
|
|