| Identification | More | [Name]
4-Acetamidophenol | [CAS]
103-90-2 | [Synonyms]
4-ACETAMIDOPHENOL
4-ACETAMINOPHENOL
4-(ACETYLAMINO)PHENOL
4'-HYDROXYACETANILIDE
4-HYDROXYACETANILIDE
ACETAMINOPHEN
ACETYL-P-AMINOPHENOL
AKOS BBS-00008094
APAP
N-(4-HYDROXYPHENYL)ACETAMIDE
N-ACETYL-4-AMINOPHENOL
N-ACETYL-P-AMINOPHENOL
P-ACETAMIDOPHENOL
P-ACETAMINOPHENOL
PARACETAMOL
P-HYDROXYACETANILIDE
RARECHEM AB PP 0382
TYLENOL
4’-hydroxy-acetanilid
4'-Hydroxyacetanilide, paracetamol | [EINECS(EC#)]
203-157-5 | [Molecular Formula]
C8H9NO2 | [MDL Number]
MFCD00002328 | [Molecular Weight]
151.16 | [MOL File]
103-90-2.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
168-172 °C(lit.)
| [Boiling point ]
273.17°C (rough estimate) | [density ]
1,293 g/cm3 | [vapor pressure ]
0.008Pa at 25℃ | [refractive index ]
1.5810 (rough estimate) | [Fp ]
11 °C | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
ethanol: soluble0.5M, clear, colorless | [form ]
Crystals or Crystalline Powder | [pka]
9.86±0.13(Predicted) | [color ]
White | [Odor]
odorless | [PH]
5.5-6.5 (H2O, 20℃)(saturated solution) | [PH Range]
5.5 - 6.5 (H?O, 20 °C) (saturated solution) | [explosive limit]
15%(V) | [Water Solubility ]
14 g/L (20 ºC) | [Usage]
Analgesic; antipyretic | [Merck ]
14,47 | [BRN ]
2208089 | [BCS Class]
3,4 | [Cosmetics Ingredients Functions]
SKIN CONDITIONING | [InChIKey]
RZVAJINKPMORJF-UHFFFAOYSA-N | [LogP]
1.098 at 25℃ | [CAS DataBase Reference]
103-90-2(CAS DataBase Reference) | [IARC]
3 (Vol. 50, 73) 1999 | [NIST Chemistry Reference]
Acetaminophen(103-90-2) | [EPA Substance Registry System]
103-90-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R36/38:Irritating to eyes and skin .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S37/39:Wear suitable gloves and eye/face protection .
S22:Do not breathe dust . | [RIDADR ]
UN 3077 9/PG III | [WGK Germany ]
1
| [RTECS ]
AE4200000
| [Autoignition Temperature]
540 °C | [TSCA ]
Yes | [HazardClass ]
9 | [PackingGroup ]
III | [HS Code ]
29242930 | [Hazardous Substances Data]
103-90-2(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 338 orally (Starmer), 500 i.p. (Dahlin, Nelson) |
| Hazard Information | Back Directory | [General Description]
Odorless white crystalline solid. Bitter taste. pH (saturated aqueous solution) about 6. | [Reactivity Profile]
4-HYDROXYACETANILIDE(103-90-2) is sensitive to light. Incompatible with strong oxidizers. . | [Air & Water Reactions]
Slightly soluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, 4-HYDROXYACETANILIDE is probably combustible. | [Description]
Acetaminophen differs from the nonsteroidal anti-inflammatory agents described in that it
is devoid of anti-inflammatory and antirheumatic properties. It was recently shown that
acetaminophen, like aspirin, inhibits cyclooxygenase action in the brain and is even
stronger than aspirin. On the other hand, the mechanism of analgesic action of acetamin�ophen is not fully clear, since it acts poorly on peripheral cyclooxygenase. | [Chemical Properties]
White Solid | [Originator]
Trigesic ,Squibb ,US ,1950 | [History]
Acetaminophen was first discovered by H. N. Morse in 1878. Although many studies on its use as an analgesic were performed, it wasn’t until 1950 that it was marketed under the name Triagesic. Today, its most common trade names are Tylenol and Panadol, but a large percentage of its sales are as a generic drug. It is the most commonly used medication for pain and fever in both the United States and Europe. It is on the World Health Organization's List of Essential Medicines.
| [Uses]
Acetaminophen is widely used as an analgesic and fever-reducing agent. Acetaminophen is
designed for moderate analgesia. It is also effective like aspirin and is used in analgesia for
headaches (from weak to moderate pain), myalgia, arthralgia, chronic pain, for oncological and
post-operational pain, etc. | [Uses]
Analgesic; antipyretic | [Uses]
antiinfectant | [Uses]
dispersing agent in liquid scintillation counting | [Uses]
manufacture of azo dyes, photographic chemicals. | [Definition]
ChEBI: Paracetamol is a member of the class of phenols that is 4-aminophenol in which one of the hydrogens attached to the amino group has been replaced by an acetyl group. It has a role as a cyclooxygenase 2 inhibitor, a cyclooxygenase 1 inhibitor, a non-narcotic analgesic, an antipyretic, a non-steroidal anti-inflammatory drug, a cyclooxygenase 3 inhibitor, a xenobiotic, an environmental contaminant, a human blood serum metabolite, a hepatotoxic agent, a ferroptosis inducer and a geroprotector. It is a member of phenols and a member of acetamides. It is functionally related to a 4-aminophenol. | [Indications]
Acetaminophen (Tylenol) is an effective antipyretic and
analgesic that is well tolerated at therapeutic doses. It
has only weak antiinflammatory activity; thus, it is not
useful in the treatment of rheumatoid arthritis and
other inflammatory conditions. | [Manufacturing Process]
About 250 ml of a reaction mixture obtained by the electrolytic reduction of
nitrobenzene in sulfuric acid solution and containing about 23 grams of paminophenol
by assay is neutralized while at a temperature of 60°C to 65°C,
to a pH of 4.5 with calcium carbonate. The calcium sulfate precipitate which
forms is filtered off, the precipitate washed with hot water at about 65°C and
the filtrate and wash water then combined. The solution is then extracted
twice with 25 ml portions of benzene and the aqueous phase is treated with
0.5 part by weight, for each part of p-aminophenol present, of activated
carbon and the latter filtered off. The activated carbon is regenerated by
treatment with hot dilute caustic followed by a hot dilute acid wash, and
reused a minimum of three times.
To the filtrate obtained, there are then added about 0.2 gram of sodium
hydrosulfite or sodium sulfite and 15.0 grams of anhydrous sodium acetate in
about 27 grams of acetic anhydride at 40°C. The reaction mixture formed is
cooled to 8°C to 10°C with stirring and held at this temperature for 60
minutes. A crystalline precipitate of about 27 grams of N-acetyl-paminophenol
is obtained melting at 169-171°C. This is equivalent to a yield of
85%.
In lieu of utilizing calcium carbonate as the neutralizing agent, calcium
hydroxide, barium hydroxide, barium chloride or other alkaline earth metal
salt or hydroxide forming an insoluble sulfate may be employed. | [Brand name]
Acephen (G & W);
Infants’ Feverall (Actavis); Injectapap (Ortho-McNeil);
Neopap (Polymedica); Tylenol (McNeil);Anacin;Crocin. | [Therapeutic Function]
Analgesic, Antipyretic | [World Health Organization (WHO)]
Paracetamol, a widely used analgesic and antipyretic is known, in
case of overdose, to cause liver damage, frequently with fatal outcome. In
recommended dosages this risk does not occur. Paracetamol is listed in the WHO
Model List of Essential Drugs. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 27, p. 1092, 1962 DOI: 10.1021/jo01050a543
Tetrahedron Letters, 22, p. 1257, 1981 DOI: 10.1016/S0040-4039(01)90289-8 | [Flammability and Explosibility]
Nonflammable | [Biological Activity]
Cyclooxygenase inhibitor; may be selective for COX-3 (IC 50 values are 460, > 1000 and > 1000 μ M for canine COX-3, and murine COX-1 and COX-2 respectively). Widely used analgesic and antipyretic agent. | [Mechanism of action]
The mechanism of action of paracetamol is not well understood, but it may
act in a similar fashion to NSAIDs, with inhibition of cyclo-oxygenase
enzymes COX-1 and COX-2 to reduce the phenoxyl radical
formation required for COX-1 and 2 activity and prostaglandin synthesis. I t
has selectivity for inhibition of prostaglandin synthesis with low
concentrations of peroxidases and arachidonic acid, but limited effect at
higher concentrations and, therefore, has limited anti-inflammatory effects.
Unlike opioids, paracetamol has no well-defined endogenous binding sites.
I n some circumstances, it may exhibit a preferential effect on COX-2
inhibition. There is growing evidence of a central antinociceptive effect of
paracetamol. It has also been found to prevent prostaglandin production at
the cellular transcriptional concentration, independent of COX activity. | [Clinical Use]
Acetaminophen is weakly acidic (pKa = 9.51) and synthesized by the acetylation of p-aminophenol. It is weakly bound
to plasma proteins (18–25%). Acetaminophen is indicated for use as an antipyretic/analgetic, particularly in those
individuals displaying an allergy or sensitivity to aspirin. It does not possess anti-inflammatory activity, but it will
produce analgesia in a wide variety of arthritic and musculoskeletal disorders. It is available in various formulations,
including suppositories, tablets, capsules, granules, and solutions. The usual adult dose is 325 to 650 mg every 4 to
6 hours. Doses of greater than 2.6 g/day are not recommended for long-term therapy because of potential
hepatotoxicity issues. Acetaminophen, unlike aspirin, is stable in aqueous solution, making liquid formulations readily
available, a particular advantage in pediatric cases. | [Side effects]
Side effects are rare and may include hematological reactions, leucopenia, agranulocytosis and other hypersensitivity reactions. Paracetamol has a narrowtherapeutic dose range and overdosage induces severe liver and renal damage via accumulation of a toxic metabolite, N-acetylbenzoquinoneimine (NABQI). Acetylcysteine or methionine, which increase glutathione conjugation of the metabolite, are used as antidote. | [Synthesis]
Acetaminophen, p-acetaminophenol (3.2.80), is synthesized by reacting
p-aminophenol with acetic anhydride [76,77].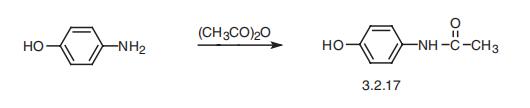
| [Environmental Fate]
Although a major part of the ingested dose of acetaminophen is
detoxified, a very small proportion is metabolized via the
cytochrome P450-mixed function oxidase pathway to a highly
reactive n-acetyl-p-benzoquinoneimine (NAPQI). The toxic
intermediate NAPQI is normally detoxified by endogenous
glutathione to cysteine and mercapturic acid conjugates and
excreted in the urine. Recent studies have shown that hepatic
P450s, CYP2E1, and to a lesser extent CYP1A2 are responsible
for conversion of acetaminophen to NAPQI. In acetaminophen
overdose, the amount of NAPQI increases and depletes
endogenous glutathione stores. Time course studies have
shown that covalent binding of reactive NAPQI and subsequent
toxicity occur only after cellular glutathione stores are
reduced by 70% or more of normal. Mitochondrial dysfunction
and damage can be seen as early as 15 min after a toxic dose in
mice, suggesting that this may be a critical to cellular necrosis.
The NAPQI is then thought to covalently bind to critical
cellular macromolecules in hepatocytes and cause cell death.
Recent proteomic studies have identified at least 20 known
proteins that are covalently modified by the reactive acetaminophen
metabolite. The resulting acetaminophen-cysteine
(APAP-CYS) protein adducts can be quantified via a highpressure
liquid chromatography coupled with electrochemical
detection (HPLC-EC). Hepatic necrosis and inflammation
develop as a consequence of hepatocellular death, which
results in development of clinical and laboratory findings
consistent with liver failure. A similar mechanism is postulated
for the renal damage that occurs in some patients following
acetaminophen toxicity. | [Metabolic pathway]
Acetaminophen (APAP) is metabolized by mice, and
nine metabolites are identified in the urine. The main
metabolites are APAP-glucuronide and 3-cysteinyl-
APAP. Hydroquinone metabolites of S-(2,5-
dihydroxyphenyl)cysteine and S-(2,5-dihydroxyphenyl)-
N-acetylcysteine result from the benzoquinone
metabolite of APAP. | [Metabolism]
acetaminophen is undergoes rapid first-pass metabolism in the GI tract primarily by conjugation reactions, with the
O-sulfate conjugate being the primary metabolite in children and the O-glucuronide being the primary metabolite in
adults. A minor, but significant, product of both acetaminophen and phenacetin is the N-hydroxyamide produced by a
CYP2E1 and CYP3A4. | [storage]
Store at RT,unstable in solution, ready to use. | [Purification Methods]
Recrystallise Paracetamol from water or EtOH. The 3,5-dinitrobenzamide complex gives orange crystals from hot H2O and has m 171.5o. [Beilstein 13 H 460, 13 I 159, 13 II 243, 13 III 1056, 13 IV 1091.] | [References]
[1] RALPH VINEGAR Jeffrey L S James F Truax. Quantitative comparison of the analgesic and anti-inflammatory activities of aspirin, phenacetin and acetaminophen in rodents[J]. European journal of pharmacology, 1976, 37 1: Pages 23-30. DOI: 10.1016/0014-2999(76)90004-2
[2] SHINSAKU KOBAYASHI HIROMU T. FEVER RESPONSES TO BACTERIAL PYROGENS IN GUINEA PIGS AND APPLICATION FOR SCREENING OF ANTIPYRETIC AGENTS[J]. Japanese journal of pharmacology, 1968, 18 1: Pages 80-85. DOI: 10.1254/jjp.18.80
[3] IRA S. LURIE. Determination of heroin and basic impurities for drug profiling by ultra-high-pressure liquid chromatography[J]. Forensic science international, 2013, 231 1: Pages 300-305. DOI: 10.1016/j.forsciint.2013.06.008
[4] MD D S B, MPH Alexander E C M Maribeth C Lovegrove MPH, MPH. Emergency Department Visits for Overdoses of Acetaminophen-Containing Products[J]. American Journal of Preventive Medicine, 2011, 40 6: Pages 585-592. DOI: 10.1016/j.amepre.2011.02.026
[5] J A MITCHELL. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase.[J]. Proceedings of the National Academy of Sciences of the United States of America, 1993, 90 24: 11693-11697. DOI: 10.1073/pnas.90.24.11693
[6] T D WARNER. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis.[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96 13: 7563-7568. DOI: 10.1073/pnas.96.13.7563
[7] BURKHARD HINZ Kay B Olga Cheremina. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man[J]. FASEB Journal, 2007, 22 2: 383-390. DOI: 10.1096/fj.07-8506com
[8] HINSON J A. Reactive metabolites of phenacetin and acetaminophen: a review.[J]. Environmental Health Perspectives, 1983, 49: 71-79. DOI: 10.1289/ehp.834971
[9] EDWARD D H?GEST?TT. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system.[J]. The Journal of Biological Chemistry, 2005, 280 36: 31405-31412. DOI: 10.1074/jbc.m501489200
[10] DERICK HAN. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria.[J]. Trends in pharmacological sciences, 2013, 34 4: 243-253. DOI: 10.1016/j.tips.2013.01.009
[11] M. TIRMENSTEIN S D N. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides.[J]. The Journal of Biological Chemistry, 1990, 138 3: 3059-3065. DOI: 10.1016/s0021-9258(19)39733-9
[12] TAMáS L?RINCZ. Ferroptosis is Involved in Acetaminophen Induced Cell Death.[J]. Pathology & Oncology Research, 2015, 21 4: 1115-1121. DOI: 10.1007/s12253-015-9946-3 |
| Questions And Answer | Back Directory | [Antipyretic analgesic]
The chemiacal name of Acetaminophen is N-(4-hydroxy phenyl) acetamide and the trade name is paracetamol belonging to acetanilide antipyretic analgesics. It was first synthesized by Morse in 1878 and first used in clinic by VonMering in 1893. It has become an over the counter drug in the USA since 1955 and our country started production at the end of the 1950’s. Acetaminophen is a white crystalline or a crystalline powder in appearance with melting point from 168℃ to 172℃, odorless, slightly bitter taste, freely soluble in hot water or ethanol, dissolved in acetone, practically insoluble in cold water and petroleum ether. It is stable below 45℃ but will be hydrolyzed into p-aminophenol when exposed to humid air, then oxidized further. The color grades gradually from pink to brown then to black, so it should be sealed and stored in a cool and dry place.Acetaminophen has the antipyretic activity by inhibiting the synthesis of hypothalamic thermoregulation prostaglandins and its strength of antipyretic effect is similar to aspirin. On the other hand, Acetaminophen can produce analgesic effect by inhibiting the synthesis of prostaglandins in the central nervous system and blocking impulses of nociceptive nerve endings, but weaker than aspirin. Compared with aspirin, Acetaminophen has minor irritation, few allergic reactions and other advantages. Its antipyretic and analgesic effect is similar to phenacetin, and the use of Acetaminophen increases due to limiting or banning using phenacetin in many countries.In clinical, it is mainly used for fever and headache caused by cold and relieving mild to moderate pain such as joint pain, muscle pain, neuralgia, migraine, dysmenorrhea, cancer pain, postoperative analgesia and so on. It can be used for patients who are allergic to aspirin, intolerant of aspirin, or unsuited for aspirin, such as patients with varicella, hemophilia and other hemorrhagic disease (patients having anticoagulant therapy included), as well as patients with slight peptic ulcer and gastritis. In addition, it also can be used for the synthesis of benorylate and used as asymmetric synthetic intermediates, photographic chemicals and stabilizer of hydrogen peroxide. | [Chemical property]
Obtain prism crystallization from ethanol. Melting point 169-171℃, relative density 1.293(21/4℃). Soluble in ethanol, acetone and hot water, difficult to dissolve in water, insoluble in petroleum ether and benzene. Odorless, bitter. The pH value of saturated aqueous solution is 5.5-6.5. | [Pharmacological Actions]
Acetaminophen is used as antipyretic analgesics. It has the antipyretic activity by means of mediated peripheral vasodilation and perspiration caused by inhibiting the cyclooxygenase which selectively inhibiting the synthesis of hypothalamic thermoregulation prostaglandins, and its strength of antipyretic effect is similar to aspirin. As a peripheral analgesic, it can produce analgesic effect by inhibiting the synthesis and release of prostaglandins and increasing pain threshold. However, its action is weaker than aspirin and it is only effective for mild to moderate pain. There is no obvious anti-inflammation effect. | [Pharmacokinetics]
The oral absorption is rapid and complete, and the peak time occurs 0.5~2h later. The plasma protein binding rate is 25%~50%. This product is equally distributed in the body, 90%~95% is metabolized in the liver and mainly excreted from the kidney combining with glucuronic acid and about 3% exits the body unchanged in the urine within 24h. Its half-life (t1/2) is 1~4h (average 2h). In case of renal insufficiency t1/2 is not affected, but t1/2 of patients with hepatic insufficiency, newborns or elderly patients may increase and t1/2 of children may decrease. It can be secreted by milk. | [Preparation method]
1. Using nitrobenzene as raw material
In the presence of concentrated sulfuric acid and sixteen alkyl methyl ammonium chloride, nitrobenzene is transformed into p-Aminophenol by catalytic hydrogenation with Pd/C as catalyst. P-acetaminophen is synthesized acetylation by one-step acylation without separation and the yield is 64.3%. The reaction is as followed:
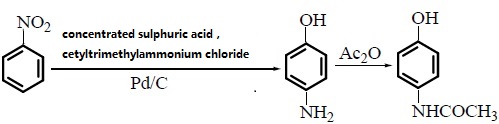
2. Using paranitrophenol as raw material
With paracetamol as raw material and Pd/C as catalyst, paracetamol is synthesized by hydroacylation on one-step method. The optimum solvent is acetic acid of which the dosage is 2 to 5 times of paranitrophenol and the yield of paracetamol is up to 95%. When Pd-La/C is used as catalyst instead, the yield can reach 97%. The reaction is as followed:
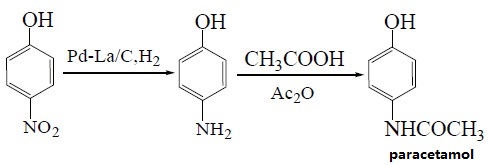
3. Using p-aminophenol as raw material
Under these conditions of using p-aminophenol and acetic anhydride as raw materials, zinc powder as the antioxidant, activated carbon as the decolorizing agent and dilute acetic acid as the reaction medium, paracetamol is synthesized by microwave irradiation technology and the yield is up to81.2%. The reaction is as followed:
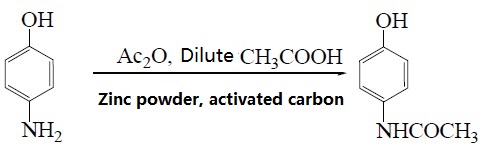
4. Using p-Hydroxyacetophenone as raw material
First oximate p-Hydroxyacetophenone and then rearrange it to obtain paracetamol by means of Beckmann. Under this method, the yield of 4-hydroxyacetophenone oxime obtained by oximating p-Hydroxyacetophenone is 93.5%. Then we use Hβ molecular sieve as catalyst and acetone as the solvent to obtain acetaminophen by rearrangement and the yield is 81.2 %. In the rearrangement reaction, acetone is used as the solvent and Al-MCM-41 molecular sieve is used as the catalyst. The yield is the highest when the content of phosphoric acid in the catalyst is 30%. The reaction is as followed:
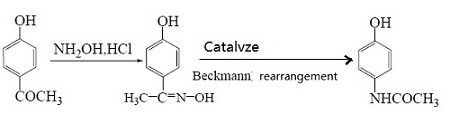
5. Using phenol as raw material
Phenol is used as the raw material and synthesizes paracetamol after acetylation, Fries rearrangement, oxime and Beckmann rearrangement. The yields are 82%, 68.6%, 50.5%,
respectively. The reaction is as followed:
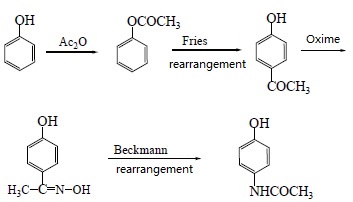 | [Usage and Dosage]
Usage
This product is antipyretic and analgesic whose international nonproprietary name is Paracetamol. It is the most common non anti-inflammatory analgesia-antipyretic drugs without anti inflammatory and anti rheumatism action. Its antipyretic effect is similar to aspirin, but analgesic effect is weak. It is the best of breed of acetanilid drugs. The product is especially suitable for patients who cannot use carboxylic acids drugs. It is used for cold and toothache. Acetaminophen is also used as organic synthesis intermediates, stabilizer of hydrogen peroxide, photographic chemicals.
Dosage
1. Oral (1) Paracetamol tablets or paracetamol capsules: adults take 300~600mg at a time and 3~4 times a day according to the need. The daily dosage should not be greater than 2g. Defervescence treatment is generally less than 3 days and the administration of pain relief lasts less than 10 days. Children take 10~15mg/kg every 4~ 6 hours. The dosage of children under the age of 12 does not exceed 5 times a day, a five-day course at most. This product should not be taken for a long time.
2. Dispersible tablets: When take tablets, disperse them in warm water dispersion. The commonly used amount of children is 10~15mg/kg every 4~ 6 hours. The dosage of children under the age of 12 does not exceed 5 times a day, a five-day course at most. Children under 3 years old cut back on the amount. | [Application in Particular Diseases]
In Osteoarthritis:
- Acetaminophen is recommended by the ACR as first-line drug therapy for pain management of OA. The dose is 325 to 650 mg every 4 to 6 hours on a scheduled basis (maximum dose 4 g/day; maximum 2 g/day if chronic alcohol intake or underlying liver disease). Comparable relief of mild to moderate OA pain has been demonstrated for acetaminophen (2.6 to 4 g/ day) compared with aspirin (650 mg four times daily), ibuprofen (1,200 or 2,400 mg daily), and naproxen (750 mg daily). However, some patients respond better to NSAIDs.
- Acetaminophen is usually well tolerated, but potentially fatal hepatotoxicity with overdose is well documented. It should be used with caution in patients with liver disease and those who chronically abuse alcohol. Chronic alcohol users (three or more drinks daily) should be warned about an increased risk of liver damage or GI bleeding with acetaminophen. Other individuals do not appear to be at increased risk for GI bleeding. Renal toxicity occurs less frequently than with NSAIDs.
| [Adverse reaction]
1. Allergic reactions: This product has less and slight side effects a dose treatment except for occasional rashes, hives and other allergic reactions. Methemoglobinemia may occur in a few cases.
2. Hepatorenal damage: A large number of long-term use, hepatorenal damages and thrombocytopenia may occur, even jaundice, oliguria, acute severe hepatitis, which could lead to coma, and death. Using at high dosage may cause nausea, vomiting, stomach pain, stomach cramps, diarrhea, anorexia, sweating, etc.
3. For children under the age of 3, the development of liver and kidney function is not mature with poor detoxification and excretory function, so they should try to avoid using this product. In addition, patients with liver and kidney insufficiency and pregnant women should use cautiously. The long-term drug users should regularly check renal function and hemogram. | [Taboo]
It is contraindicated in patients allergic to the product and patients with severe liver and kidney function deficiency. | [Notes]
- Allergies are disabled. Patients who are allergic to aspirin do not have allergic reactions generally. However, it has been reported that a small number of patients with asthma caused by aspirin-sensitivity can have an episode of bronchospasm after taking drugs.
- This product may increase the risk of liver toxicity when patients suffer from alcohol poisoning, liver disease or viral hepatitis, so it should be used with caution. Patients with renal insufficiency take a lot of products regularly, leading to increasing risk of renal toxicity, so they should be careful. It is contraindicated in patients with severe liver and kidney function deficiency.
- When taking the drug for pain, it is not allowed to take for more than 40 consecutive days. Antipyretic treatments shall not exceed 3 days, unless doctors tell you otherwise. After taking this product, patients should immediately stop taking medicine when symptoms of erythema or edema occur. This product is only a drug for symptomatic treatment, it is necessary to take other treatments to relieve reasons of pain or fever at the same time when taken.
- This product can be passed through the placenta and secreted in milk, so pregnant women and lactating women are not recommended to use. For children under the age of 3, the development of liver and kidney function is not mature with poor detoxification and excretory function, so they should try to avoid using this product. Because the development of liver and kidney function declines, t1/2 of elderly patients may increase leading to adverse reactions easily. Patients should take with caution or take a smaller amount of use appropriately.
- The interferences of diagnosis: ① Glucose measurement, falsely low values are measured by glucose oxidase/peroxidase methods, but no effect occurs when measured by hexokinase /6-dehydrogenase methods; ② Assays for uric acid of serum, falsely high values are measured by phosphotungstic acid method; ③ Determination of urinary 5-hydroxyindoleacetic acid (5-HIAA), falsely positive results are obtained in a screening test with nitroso naphthol reagent, but quantitative test is not affected; ④Liver function tests, prothrombin time, serum bilirubin, lactic dehydrogenase and serum aminotransferase can be increased due to high doses or long-term use.
- In case of large dosage, promote vomiting timely and give antagonists named N-acetylcysteine (140mg/kg orally given at the beginning, then 70mg/kg, take 1 times every 4h, 17 times; it can be given intravenously when serious, the drug can be dissolved in 5% 200 ml glucose injection and used through intravenous drip) or take methionine orally, which has a protective effect on the liver. Do not give activated carbon, because it can affect the absorption of drugs. The antagonist should be applied as soon as possible because the effect is satisfactory in 12 h but the effect is worse over 24 h. In the treatment, it is best to monitor the blood concentration and give other therapies, such as hemodialysis or hemofiltration.
| [Drug interactions]
- For patients with chronic alcohol ingestion or other liver enzyme inducers, especially barbiturates or anticonvulsants, when taking long-term or a large-scale use of this product, they may have a higher risk of liver toxicity.
- When combined with chloramphenicol, this product can prolong the latter t1/2 and enhance its toxicity.
- When combined with anticoagulant drugs, this product can increase the anti-blood-clotting effect. So it is necessary to adjust the dosage of anticoagulant drugs.
- When combining long-term large quantities of Acetaminophen with aspirin or other non-steroidal anti-inflammatory drugs, it will increase the risk of renal toxicity.
- When combined with the antiviral drug, zidovudine, it can increase the toxicity. We ought to avoid using at the same time.
| [Administration nursing care point]
- Nurse according to the general principles of analgesia-antipyretic drugs.
- The caregiver should exhort the patient to pay attention to the following things during medication period: ①No drinking, drinking may aggravate the liver toxicity of this product; ②Drink plenty of water to reduce the concentration of drug in renal tubules and reduce the occurrence of “analgesic nephropathy”; ③When taking chewing chips, chew them up; ④No unauthorized use other NSAIDS or compound preparation containing NSAIDS at the same time to avoid increasing the renal toxicity.
- In a poisoning caused by this product, we should give patients oral antagonists, acetylcysteine (Tan Yijing) as soon as possible, not oral activated carbon, because the latter can affect the absorption of antagonists. Initial dose of acetylcysteine is 140 mg/kg, add 70 mg/kg every 4 h, 17 times totally. Intake: configure acetylcysteine into a 5% solution or add in triple drinks and take after shaking well to avoid fetid odors and irritations. For the occurrence of vomiting within 1 h after medication, resupply, if necessary, take nasal or rectal administration. In severe cases, the drug can be dissolved in 5% 200 ml glucose injection and used through intravenous drip. The antagonist should be applied as soon as possible because the effect is satisfactory in 12 h but the effect is worse over 24 h. In the treatment, it is best to monitor the blood concentration and give other therapies, such as hemodialysis or hemofiltration.
| [Usage]
Organic synthesis intermediates, stabilizer of hydrogen peroxide, photographic chemicals, non anti-inflammatory analgesia-antipyretic drugs. | [Production]
Produced by acetylation of p-aminophenol.
Method 1: add p-aminophenol into dilute acetic acid, then add glacial acetic acid, heat up to 150℃and react for 7h, add acetic anhydride and react for 2h, check the end point and cool to 25℃ after the acceptance, shake it and filter, water until no acetic acid flavor exists, dry to get crude products.
Method 2: distill p-aminophenol, acetic acid and acid industrial containing more than 50% acid together, the speed of distilling dilute acid for is 1/10 of the total distillate in one hour, check the residue of p-aminophenol less than 2.5% aminophenol by sampling inspection when inner temperature rises up to 130℃, add dilute acid (content of more than 50%), cool to get crystallization. After shaking and filter, first use a small amount of dilute acid to wash, and then use a large number of water till filtrate is near colourless to get crude products. The yield of method 1 is 90%, but the yield of method 2 is 90-95%. Refining methods: add the crude product when the water is heated to near boiling. Heat up to the total dissolution, add activated carbon soaked in water, use dilute acetic acid to adjust till pH=4.2-4.6, boil for 10min. Filter press, add a small amount of sodium bisulfite into the filtrate. Cool to below 20℃, separate crystals out. After shaking and filter, wash and dry to get active ingredients, paracetamol finished products.
Other methods of production are as followed:
(1) p-nitrophenol is reduced by zinc in acetic acid, and acetaminophen is obtained by acetylation at the same time;
(2) put the hydrazone generated from p-hydroxyacetophenone in acid solution containing sulfuric acid, and then add sodium nitrite to get acetaminophen by renversement.
|
|
|