| Identification | More | [Name]
Ferric chloride | [CAS]
7705-08-0 | [Synonyms]
BOD IRON(III) CHLORIDE SOLUTION D
FERRIC CHLORIDE
FERRIC CHLORIDE CS
FERRIC CHLORIDE OXIDIZING
FERRIC CHLORIDE REAGENT
FERRIC CHLORIDE SOLUTION
FERRIC CHLORIDE SOLUTION R1
FERRIC PERCHLORIDE
FERRIC TRICHLORIDE
Ferric trichloride,solution
IRON(+3)CHLORIDE
IRON ATOMIC SPECTROSCOPY STANDARD
IRON CHLORIDE
IRON(III) CHLORIDE
IRON (III) CHLORIDE ON SILICA
IRON TRICHLORIDE
TDA REAGENT
TRYPTOPHAN DEAMINASE REAGENT
Chlorure perrique
chlorureferrique | [EINECS(EC#)]
231-729-4 | [Molecular Formula]
Cl3Fe | [MDL Number]
MFCD00011005 | [Molecular Weight]
162.2 | [MOL File]
7705-08-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Ferric Chloride is a black-brown, dark-green,
or black crystalline solid. | [Melting point ]
304 °C(lit.) | [Boiling point ]
316 °C | [bulk density]
1000kg/m3 | [density ]
2,804 g/cm3 | [vapor density ]
5.61 (vs air)
| [vapor pressure ]
1 mm Hg ( 194 °C)
| [refractive index ]
n20/D1.414 | [Fp ]
316°C | [storage temp. ]
2-8°C | [solubility ]
H2O: soluble
| [form ]
powder
| [color ]
Yellow | [Specific Gravity]
2.804 | [PH]
1 (200g/l, H2O, 20℃) | [Stability:]
Stable. Very sensitive to moisture. Incompatible with strong oxidizing agents; forms explosive mixtures with sodium, potassium. Hygroscopic. | [Water Solubility ]
920 g/L (20 ºC) | [Crystal Structure]
BiI3 type | [Sensitive ]
Hygroscopic | [crystal system]
Three sides | [Merck ]
14,4019 | [Space group]
R3 | [Lattice constant]
| a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.606 | 0.606 | 1.741 | 90 | 90 | 120 | 0.5529 |
| [Exposure limits]
ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 | [Cosmetics Ingredients Functions]
ASTRINGENT | [InChI]
1S/3ClH.Fe/h3*1H;/q;;;+3/p-3 | [InChIKey]
RBTARNINKXHZNM-UHFFFAOYSA-K | [SMILES]
Cl[Fe](Cl)Cl | [Uses]
Treatment of sewage and industrial wastes;
etching agent for engraving, photography, and
printed circuitry; condensation catalyst in FriedelCrafts reactions; mordant; oxidizing, chlorinating,
and condensing agent; disinfectant; pigment; feed
additive; wat | [CAS DataBase Reference]
7705-08-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Ferric chloride(7705-08-0) | [EPA Substance Registry System]
7705-08-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C,Xn,Xi | [Risk Statements ]
R41:Risk of serious damage to eyes.
R38:Irritating to the skin.
R22:Harmful if swallowed.
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 2582 8/PG 3
| [WGK Germany ]
1
| [RTECS ]
LJ9100000
| [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
28273990 | [Storage Class]
8B - Non-combustible corrosive hazardous materials | [Hazard Classifications]
Acute Tox. 4 Oral
Eye Dam. 1
Met. Corr. 1
Skin Irrit. 2 | [Safety Profile]
Poison by ingestion and
intravenous routes. Experimental
reproductive effects. Corrosive. Probably an
eye, skin, and mucous membrane irritant.
Mutation data reported. Reacts with water
to produce toxic and corrosive fumes.
Catalyzes potentially explosive
polymerization of ethylene oxide, chlorine +
monomers (e.g., styrene). Forms shock sensitive explosive mixtures with some
metals (e.g., potassium, sodium). Violent
reaction with all$ chloride. When heated to
decomposition it emits highly toxic fumes of
HCl. | [Hazardous Substances Data]
7705-08-0(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 316 mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Chlorine-->Iron-->Oxygen-->Bifenthrin-->Iron chloride hexahydrate-->Ferrous chloride-->Free and total Chlorine, ion specific meter | [Preparation Products]
2,2'-Bipyridine-->Propanil-->Ferrous lactate-->VINYL CHLORIDE-->2,3-Dichloro-1,4-naphthoquinone-->5-amino-1,3,4-thiadiazole-2-carboxylic acid-->trans-1,4-Dichloro-2-butene-->6-Methylpyridine-2-carbonitrile-->2-Chloro-6-nitrotoluene-->2-Chloro-4-nitrobenzoic acid-->ETHOPERMETHRIN,95%-->3-Chloro-4-methylaniline-->2,3,4-Trimethoxyphenylboronic acid-->BUTRALIN-->EDTA ferric sodium salt-->Musk ketone-->Ferric pyrophosphate-->2-Methoxyphenylacetone-->Stevioside-->5-AMINO-1,3,4-THIADIAZOLE-2-CARBOXYLIC ACID ETHYL ESTER-->3,4-Hexanedione-->(1S)-(+)-3-Carene-->Seratrodast-->5,12-DIHYDRO-5,7,12,14-TETRAZAPENTACENE-->polythiniren-->3-Chlorobenzotrifluoride-->Iron naphthenate-->3-TRIFLUOROMETHYL BENZOTRICHLORIDE-->2,5-DICHLOROBENZOYL CHLORIDE-->6-METHOXY-4-METHYLQUINOLINE-->OVEX-->poly (3-alkyl thiophene) fiber-->SOLVENT BLACK 5-->Thiophene-3,4-dicarboxylic acid-->4'-(Trifluoromethoxy)acetophenone-->3-AMINO-PHENAZIN-2-OL-->2,3-DIAMINOPHENAZINE-->2,5-DICHLORO-P-XYLENE-->FERRIC PHOSPHATE TETRAHYDRATE-->MAFA |
| Hazard Information | Back Directory | [General Description]
FERRIC CHLORIDE(7705-08-0) is an orange to brown-black solid. FERRIC CHLORIDE(7705-08-0) is slightly soluble in water. FERRIC CHLORIDE(7705-08-0) is noncombustible. When wet FERRIC CHLORIDE(7705-08-0) is corrosive to aluminum and most metals. Pick up and remove spilled solid before adding water. FERRIC CHLORIDE(7705-08-0) is used to treat sewage, industrial waste, to purify water, as an etching agent for engraving circuit boards, and in the manufacture of other chemicals. | [Reactivity Profile]
Alkali metal hydroxides, acids, anhydrous chlorides of iron, tin, and aluminum, pure oxides of iron and aluminum, and metallic potassium are some of the catalysts that may cause ethylene oxide to rearrange and polymerize, liberating heat, [J. Soc. Chem. Ind. 68:179(1949)]. Explosions occur , although infrequently, from the combination of ethylene oxide and alcohols or mercaptans, [Chem. Eng. News 20:1318(1942)]. Allyl chloride may polymerize violently under conditions involving an acid catalyst, such as sulfuric acid, ferric chloride, aluminum chloride, Lewis acids, and Ziegler type catalysts (initiators), [Ventrone (1971)]. | [Air & Water Reactions]
Very hygroscopic. Slightly water soluble, where a 0.1M solution has a pH of 2.0. | [Hazard]
Toxic by ingestion, strong irritant to skin
and tissue. | [Health Hazard]
Inhalation of dust may irritate nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes. Prolonged contact with skin causes irritation and burns. | [Potential Exposure]
Iron chloride is used to treat sewage
and industrial waste. It is also used as an etchant for photo engraving and rotogravure; in textiles; photography; as a
disinfectant; as a feed additive. | [Fire Hazard]
Special Hazards of Combustion Products: Irritating hydrogen chloride fumes may form in fire. | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim ingested or inhaled the substance; give artificial respira tion with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with run ning water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves.
| [Shipping]
UN1773 Ferric chloride, anhydrous, Hazard
class: 8; Labels: 8-Corrosive material. UN2582 Ferric chlo ride, solution, Hazard class: 8; Labels: 8-Corrosive
material | [Incompatibilities]
Aqueous solutions are a strong acid.
Violent reaction with bases, allyl chloride; sulfuric acid;
water. Shock- and friction-sensitive explosive material
forms with potassium, sodium and other active metals.
Attacks metals when wet. | [Description]
Ferric chloride (iron(IH)chloride, FeCl3, CAS No. 7705-08-0) may be prepared from iron and chlorine or from ferric oxide and hydrogen chloride. The pure material occurs as hydroscopic, hexagonal, dark crystals. Ferric chloride hexahydrate (iron(III)chloride hexahydrate, FeCl3*6H2O, CAS No. 10025-77-1) is readily formed when ferric chloride is exposed to moisture.
| [Chemical Properties]
Ferric Chloride is a black-brown, dark-green,
or black crystalline solid. | [Chemical Properties]
Ferric chloride,FeCl3, is a brown crystalline solid and is soluble in water,alcohol,and glycerol. It is also known as anhydrous ferric chloride,ferric trichloride, Flores martis,and iron chloride. Ferric chloride is used as a coagulant for sewage and industrial wastes, as an oxidizing and chlorinating agent,as a disinfectant, in copper etching, and as amordant. In addition, this compound is employed in the ferric chloride test,which is used to assess the relative corrosion resistance of stainless and nickel-base alloys. The ferric chloride test has been shown to be an appropriate measure of the suitability of such alloys for service in paper mill bleach plants and seawater.
| [Waste Disposal]
Neutralize with lime or soda
ash and bury in an approved landfill. | [Definition]
ChEBI: Iron trichloride is an iron coordination entity. It has a role as a Lewis acid and an astringent. | [Flammability and Explosibility]
Nonflammable | [reaction suitability]
reagent type: catalyst
core: iron | [Industrial uses]
Ferric chloride (FeCl3) is obtained by an iron chlorination method at a temperature of
600–700 °C. Very limited data are available on the use of ferric chloride in the mineral
processing industry. Ferric chloride has a depressing effect on barite and can be used
in barite–celestite separation. It was also evaluated as a depressant during niobium–
zirconium separation. In general, ferric and ferrous compounds are not selective
depressants and in many cases are detrimental for flotation of oxidic and industrial
minerals as in the case of anionic flotation, fatty acid, iron complexes or oleate iron
complexes. | [storage]
(1) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(2) Color Code—Blue: Health Hazard/Poison: Store in asecure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Iron chloride must be stored in tightly closed containers toavoid contact with sulfuric acid, sodium, potassium, allylchloride, and water, since violent reactions occur and toxicvapors may be produced. | [Purification Methods]
Sublime it at 200o in an atmosphere of chlorine. It is an “iron-black” coloured powder with green irridescence. Store it in a weighing bottle inside a desiccator as it absorbs moisture from air to form the yellow hexahydrate (see next entry). [Tarr Inorg Synth III 191 1950, Pray Inorg Synth V 153 1957, Epperson Inorg Synth VII 163 1963.] | [Structure and conformation]
The crystalline solid has a semicovalent layer structure with hexagonal packing of
chloride ions, each iron atom being surrounded octahedrally by six chlorines in a BiI3 type
structure. The dimers in the vapour phase have a structure similar to that of Al2Cl6
with the iron atoms surrounded by chlorines in a roughly tetrahedral fashion. The
magnetic properties of iron(III) chloride in its different environments have been investigated
extensively. The magnetic moment at 290°K is 5-73 B.M. and is independent of the
field strength. In aqueous hydrochloric acid the room temperature moment is 5-94 B.M.
and the hexahydrate has a similar moment (5-95 B.M.). |
| Questions And Answer | Back Directory | [Physical properties]
Ferric chloride, solution appears as a colorless to light brown aqueous solution that has a faint hydrochloric acid odor. Highly corrosive to most metals and probably corrosive to tissue. Noncombustible. Used in sewage treatment and water purification.

Ferric chloride is an dark brown hexagonal crystals; hygroscopic; density 2.898g/cm3; melts at 306°C; decomposes at 315°C; highly soluble in water (74.4g/100g water at 0°C); very soluble in alcohol, ether and acetone. The hexahydrate is brownish-yellow crystalline mass; deliquesces; melts at 37°C; vaporizes around 280°C; highly soluble in water (92g/100g water at 20°C); very soluble in organic solvents such as ethanol, ether and acetone. | [Uses]
Iron(III) chloride occurs naturally as the mineral molysite. The compound is widely used to prepare a number of iron(III) salts. Also, it is applied in sewage and industrial waste treatment processes. It also is used in the manufacture of dyes, pigments and inks; as a chlorinating agent; and as a catalyst in chlorination reactions of aromatics.
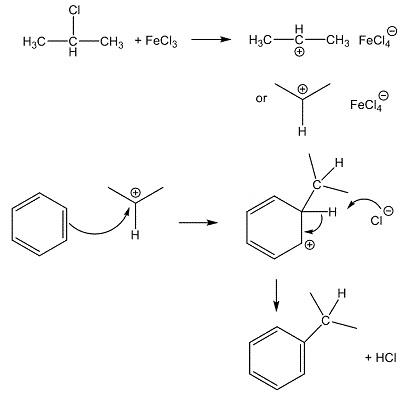
In the laboratory, anhydrous Ferric chloride(FeCl3)can be used as an alternative to anhydrous AlCl3 in Friedel-Crafts reactions, like the alkylation or acylation of aromatic rings. Being a small, highly charged metal, it polarises reagents like halogenoalkanes (alkyl halides), which generates carbocations (carbenium ions) that then attack benzene rings, as in the synthesis here. | [Production Methods]
Iron(III) chloride forms passing chlorine gas over iron filings at 350°C:
2Fe + 3Cl2 → 2FeCl3
It also forms heating iron(III) oxide with HCl at elevated temperatures:
Fe2O3 + 6HCl → 2FeCl3 + 3H2O
The product may be sublimed in a stream of chlorine to give high purity grade iron(III) chloride.
The anhydrous chloride also may be made by heating the hexahydrate, FeCl3•6H2O, with thionyl chloride:
FeCl3•6H2O + 6SOCl2 → FeCl3 + 12HCl + SO2
| [Application]
The following list represents some of the most common and largest applications of Ferric Chloride:
As a purifying agent in water supply and as a coagulant in municipal and industrial wastewater treatment. In this application, Ferric chloride’s rapid hydrolysis in water makes it an ideal flocculating and precipitating agent. The ferric hydroxide (Fe[OH]3) produced forms flocs (small clumps or tufts) that adsorb suspended particles of various materials (e.g., colloids, clays and bacteria). The clumps, with the adsorbed matter, are then allowed to settle for easy removal. Ferric chloride forms precipitates with hydrogen sulfide (H2S), phosphate (PO4), arsenic as arsenate (AsO4) and hydroxide alkalinity (OH).
As an oxidant in indigo blue dyestuff production.
As an etching medium in producing printed circuit boards (PCBs).
As a catalyst for the reaction of ethylene with chlorine, forming ethylene dichloride (1,2-dichloroethane), an important commodity chemical, which is mainly used for the industrial production of vinyl chloride, the monomer for making PVC.
www.marchpump.com |
|
|