| Identification | More | [Name]
Flumazenil | [CAS]
78755-81-4 | [Synonyms]
4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-, ETHYL ESTER
8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-4H-IMIDAZO[1,5-A][1,4]BENZO-DIAZEPINE-3-CARBOXYLIC ACID ETHYL ESTER
8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID ETHYL EST
ethyl 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4h-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate
FLUMAZENIL
RO 15-1788
Flumazenil,Flumazepil
FlumazenilUsDmf
ANEXATE
Flumazepil
4H-Imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, 8-fluoro-5,6-dihydro-5-methyl-6-oxo-, ethyl ester
8-Fluoro-5,6-dihydro-5-methyl-6-oxo-4H-Imidazo[1,5-a][1,4]benzo-diazepine-3-carboxylic acid ethyl ester
FMZ
Ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-α][1,4]benzodiazepine-3-carboxylate
Lanexat
Mazicon
Rcr-15-1788
Romazicon
Ethyl 8-fluoro-5-methyl-5,6-dihydro-6-oxo-4H-imidazo(1,5-a)(1,4)benzodiazepine-3-carboxylate
8-Fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazol[1,5-a][1,4]benzodiazepine-3-carboxylic acid ethyl ester | [EINECS(EC#)]
616-650-9 | [Molecular Formula]
C15H14FN3O3 | [MDL Number]
MFCD00242764 | [Molecular Weight]
303.29 | [MOL File]
78755-81-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Colourless crystals | [Melting point ]
201-203°C | [Boiling point ]
528.0±50.0 °C(Predicted) | [density ]
1.39±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
Soluble in DMSO to 25mM | [form ]
solid
| [pka]
0.86±0.20(Predicted) | [color ]
white
| [Water Solubility ]
128 mg/L | [Merck ]
14,4135 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | [Major Application]
pharmaceutical (small molecule) | [InChI]
InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | [InChIKey]
OFBIFZUFASYYRE-UHFFFAOYSA-N | [SMILES]
N12C=NC(C(OCC)=O)=C1CN(C)C(=O)C1=CC(F)=CC=C12 | [CAS DataBase Reference]
78755-81-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Flumazenil(78755-81-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
2
| [RTECS ]
NI2922170
| [HS Code ]
2933997500 | [Storage Class]
11 - Combustible Solids | [Toxicity]
LD50 in mice, rats (mg/kg): 4000, 1360 i.p.; 4300, 6000 orally (Hunkeler) |
| Hazard Information | Back Directory | [Description]
Flumazenil is a benzodiazepine antagonist useful as a fast-acting antidote in the treatment
of benzodiazepine intoxication, and in reversing the central sedative effects of
benzodiazepines during anesthesia. | [Chemical Properties]
Colourless crystals | [Originator]
Hoffmann-La Roche (Switzerland) | [Uses]
A benzodiazepine antagonist | [Uses]
benzodiazepine antagonist sedation reversal drug | [Uses]
beta-blocker, antihypertensive | [Uses]
Imidazodiazepine which selectively blocks the central effects of classic benzodiazepines. It is used as benzodiazepine antagonist. | [Definition]
ChEBI: An organic heterotricyclic compound that is 5,6-dihydro-4H-imidazo[1,5-a][1,4]benzodiazepine which is substituted at positions 3, 5, 6, and 8 by ethoxycarbonyl, methyl, oxo, and fluoro groups, respectively. It is used as an
ntidote to benzodiazepine overdose. | [Preparation]
The Synthesis of Flumazenil
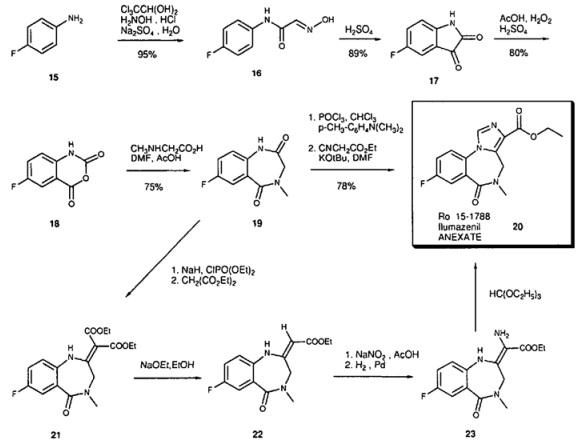
Starting with 4-fluoroaniline (15) the isatin 17 is synthesized via the Sandmeyer synthesis; isatin is then oxidized with peracetic acid to the isatoic anhydride 18. Reaction with sarcosine in DMF leads to the benzodiazepine-2,5-dione 19. This is converted to the iminochloride by reaction with POCI3 . In the key step the imidazoester is built up by reaction with deprotonated ethyl isocyanoacetate [8]. Since ethyl isocyanoacetate is not very stable, an alternative synthesis based on the synthesis of midazolam was developed for large scale-production. Tnthis synthesis diethylmalonate is used. The diester 21 is then transformed to the monoester 22 hy deethoxycarbonylation. Nitrosation and catalytic reduction lead to the amino compound 23. The final carbon atom is introduced by reaction with the orthoester. | [Manufacturing Process]
24 g (132.5 mmol) of 5-fluoroisatoic acid anhydride are dissolved in 140 ml of
dimethyl sulphoxide and treated with 11.8 g (132.5 mmol) of sarcosine. The
solution is stirred at 100°C until the gas evolution ceases (duration: ca 1.5 h)
and subsequently poured into ca 1.2 L of water. After stirring for 10 min, a
solid crystallizes out. The crystals are filtered off under suction, washed with 1
L of water and dried. There is obtained 7-fluoro-3,4-dihydro-4-methyl-2H-1,4-
benzodiazepine-2,5(1H)-dione of melting point 262°-263°C.
A solution of 6.5 g (32 mmol) of 7-fluoro-3,4-dihydro-4-methyl-2H-1,4-
benzodiazepine-2,5(1H)-dione in 30 ml of dry dimethylformamide is treated
with 4.3 g (38 mmol) of potassium t-butylate under an argon atmosphere.
The temperature thereby rises to 35°C. After 10 min, the mixture is cooled to
-30°C and 5.8 g (34 mmol) of diethylchlorophosphate are added dropwise
thereto at -30°C to -20°C. The solution is subsequently stirred at -200°C for
10 min.
Separately, 4 g (35 mmol) of potassium tert-butylate are dissolved in 10 ml of
dimethylformamide and treated at ca. -40°C with 4 g (35 mmol) of ethyl
isocyanoacetate. This solution is added dropwise at -10°C to -20°C to the
mixture obtained according to the preceding paragraph. The resulting mixture
is then stirred without cooling for 1 h, 3.2 ml of glacial acetic acid are added
thereto, the mixture is poured into ca. 400 ml of water and extracted three
times with 150 ml of ethyl acetate each time. The combined organic extracts
are washed five times with 200 ml of water each time, dried over magnesium
sulfate and evaporated. From the oily residue there is obtained, by column
chromatography on silica gel and subsequent recrystallisation from ethyl
acetate and ether, ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-
a][1,4]benzodiazepine-3-carboxylate of melting point 199°-200°C. | [Brand name]
Romazicon (Roche);Anexate. | [Therapeutic Function]
Benzodiazepine receptor antagonist, Anticonvulsant | [Biological Activity]
Benzodiazepine antagonist, non-selective for α 1, α 2, α 3 or α 5-containing GABA A receptors. Centrally active upon systemic administration in vivo . | [Pharmacokinetics]
Flumazenil is a competitive antagonist at the GA BAA benzodiazepine
binding site for all other ligands. I t rapidly reverses the CN S and dangerous
physiological effects of benzodiazepines following iatrogenic overdose or
deliberate self-harm. I t has no effect on benzodiazepine metabolism.
Flumazenil is rapidly cleared from plasma and metabolised by the liver and
has a very short elimination half-life (<1h). Its duration of action depends on
the dose administered and the duration of action of the drug to be
antagonised; repeated administration or infusions may be necessary. | [Clinical Use]
Reversal of sedative effects of benzodiazepines in
anaesthetic, intensive care, and diagnostic procedures | [Veterinary Drugs and Treatments]
Flumazenil may be useful for the reversal of benzodiazepine effects
after either therapeutic use or overdoses. Flumazenil may be of
benefit in the treatment of encephalopathy in patients
with severe
hepatic failure. | [Drug interactions]
Potentially hazardous interactions with other drugs
None known | [Metabolism]
Flumazenil is extensively metabolised in the liver. The carboxylic acid metabolite is the main metabolite
in plasma (free form) and urine (free form and its
glucuronide). This main metabolite showed no
benzodiazepine agonist or antagonist activity in
pharmacological tests.Flumazenil is almost completely (99%) eliminated by
non-renal routes. Practically no unchanged flumazenil
is excreted in the urine, suggesting complete metabolic
degradation of the drug. Elimination of radiolabelled drug
is essentially complete within 72 hours, with 90-95% of
the radioactivity appearing in urine and 5-10% in the
faeces. | [storage]
+4°C (desiccate) | [Mode of action]
Flumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on the central nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex. In animal experiments the effects of compounds showing no affinity for the benzodiazepine receptor, e.g. barbiturates, ethanol, meprobamate, GABA mimetics, adenosine receptor agonists and other agents were not affected by flumazenil, but those of nonbenzodiazepine agonists of benzodiazepine receptors, such as cyclopyrrolones (e.g. zopiclone) and triazolopyridazines were blocked. | [References]
References/Citations: |
|
|