2004-07-1
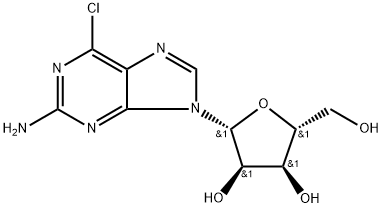 2004-07-1 结构式
2004-07-1 结构式
基本信息
6-氯鸟嘌呤核甙
6-氯鸟嘌呤核苷
6-氯腺苷
6-氯鸟嘌呤苷
2-AMINO-6-CHLOROPURINE-9-BETA-D-RIBOSIDE
2-AMINO-6-CHLOROPURINE-9-RIBOSIDE
2-AMINO-6-CHLOROPURINE RIBOSIDE
6-CHLOROADENOSINE
6-CHLOROGUANINE RIBOSIDE
6-CHLOROGUANOSINE
6-chloro-9-beta-D-ribofuranosyl-9H-purin-2-amine
2-Amino-6-chloro-9H-purine-9-riboside
2-Amino-6-chloropurine-9-b-D-riboside
2-Amino-6-chloro-9-(b-D-ribofuranosyl)purine
6-Chloroguanosine2
2-AMINO-6-CHLOROPURINE RIBOSIDE 97% (HPLC)
2-AMINO-6-CHLOROPURINE RIBOSIDE (6-CHLOROGUANOSINE)
9H-PURIN-2-AMINE, 6-CHLORO-9-.BETA.-D-RIBOFURANOSYL-
2-Amino-6-chloropurine-9--D-riboside
6-Chloroguanine nucleoside
(-)-2-Amino-6-chloropurine riboside, 6-Chloroguanine riboside
1β-(2-Amino-6-chloro-9H-purine-9-yl)-1-deoxy-β-D-ribofuranose
2-Amino-6-chloro-9-β-D-ribofuranosyl-9H-purine
物理化学性质
制备方法
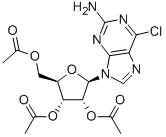
16321-99-6

2004-07-1
一般步骤:将(2R,3R,4R,5R)-2-(乙酰氧基甲基)-5-(2-氨基-6-氯-9H-嘌呤-9-基)四氢呋喃-3,4-二基二乙酸酯(1.56g,3.64mmol)溶于丙酮(10mL)中,加入磷酸盐缓冲液(58mL,pH8)和Novozyme(1.6g)。将反应混合物在60℃下搅拌8天。反应完成后,过滤去除酶,并用乙醇(50mL)和丙酮(50mL)洗涤。将滤液减压浓缩,所得粗产物通过硅胶柱色谱纯化(洗脱剂:CH2Cl2:MeOH,85:15),得到(2R,3R,4S,5R)-2-(2-氨基-6-氯-9H-嘌呤-9-基)-5-(羟甲基)四氢呋喃-3,4-二醇(0.61g,收率55%),为淡蓝色粉末。1H NMR (400 MHz, DMSO-d6) δ 8.38 (s, 1H, H-8), 6.99 (s, 1H, NH2), 5.81 (d, J = 5.80 Hz, 1H, H-1'), 5.49 (br s, 1H, OH-2'), 5.19 (br s, 1H, OH-3'), 5.05 (t, J = 5.24 Hz, 1H, OH-5'), 4.47 (t, J = 4.85 Hz, 1H, H-2'), 4.20-4.02 (m, 1H, H-3'), 3.90 (q, J = 3.98 Hz, 1H, H-4'), 3.69-3.60 (m, 1H, H2-5'), 3.59-3.52 (m, 1H, H2-5'); 13C NMR (100 MHz, DMSO-d6) δ 160.5, 154.7, 150.2, 141.9, 124.2, 87.4, 86.0, 74.2, 70.9, 61.9; 高分辨率ES-MS m/z 302.0649 ([M + H]+, C10H1335ClN5O4计算值302.0651)。
参考文献:
[1] Advanced Synthesis and Catalysis, 2012, vol. 354, # 1, p. 96 - 104
[2] Carbohydrate Research, 2017, vol. 452, p. 91 - 96
[3] Canadian Journal of Chemistry, 2001, vol. 79, # 12, p. 1881 - 1886
[4] Tetrahedron Letters, 2005, vol. 46, # 47, p. 8225 - 8228
[5] ChemMedChem, 2011, vol. 6, # 6, p. 1074 - 1080
