4016-63-1
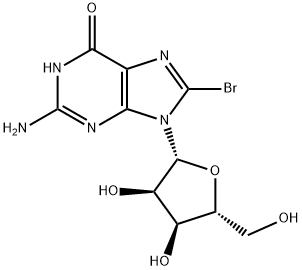 4016-63-1 结构式
4016-63-1 结构式
基本信息
8-溴鸟苷
8-溴鸟嘌呤核苷
8-溴鸟苷水合物
8-BROMOGUANOSINE
8-Bromo-D-guanosine
8-BROMOGUANOSINE, HPLC PURIFIED, 98% PURE WITH HPLC UV CHROMATOGRAM
物理化学性质
制备方法
118-00-3

4016-63-1
向鸟苷(4.0 g,14.4 mmol)在水(100 mL)中的悬浮液中逐滴加入溴(0.8 mL,14.6 mmol),控制加入速度使反应混合物在每次加入后恢复至无色。当反应混合物因残留溴而呈现微黄色时终止加溴。迅速抽滤收集无色固体产物,依次用60 mL冷水和30 mL冷丙酮洗涤。将粗产物溶于150 mL热水中进行重结晶,随后于60℃下真空干燥6小时。最终获得4.4 g(11.8 mmol,收率85%)目标化合物13,为无色针状晶体,熔点198-200℃。核磁共振数据如下:1H NMR (400 MHz, DMSO-d6) δ: 10.82 (1H, s), 6.50 (2H, s), 5.69 (1H, d, J = 6.4 Hz), 5.45 (1H, d, J = 6.0 Hz), 5.09 (1H, d, J = 5.2 Hz), 5.02 (1H, dd, J = 6.0, 11.6 Hz), 4.92 (1H, t, J = 6.0 Hz), 4.14 (1H, m), 3.86 (1H, m), 3.66 (1H, m), 3.52 (1H, m); 13C NMR (100 MHz, DMSO-d6) δ: 155.5, 153.4, 152.0, 121.2, 117.5, 89.7, 85.8, 70.5, 70.3, 62.0。
参考文献:
[1] Photochemistry and Photobiology, 2018, vol. 94, # 4, p. 677 - 684
[2] Nucleosides, Nucleotides and Nucleic Acids, 2012, vol. 31, # 10, p. 707 - 719
[3] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2001, vol. 40, # 5, p. 382 - 385
[4] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2002, vol. 41, # 2, p. 379 - 383
[5] Tetrahedron Letters, 2016, vol. 57, # 27-28, p. 2949 - 2953
