65564-05-8
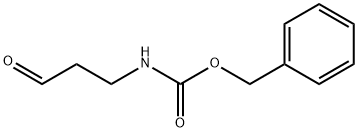 65564-05-8 结构式
65564-05-8 结构式
基本信息
3-(CBZ-氨基)丙醛
CBZ-BETA-丙氨醛
N-CBZ-3-氨基丙醛
3-苄氧基羰酰胺-1-丙醇
N-苄氧羰基-3-氨基丙醛
3-[(苄氧羰基)胺]丙醛
3-[(羰苄氧基)氨基]丙醛
3-[(苄氧基羧基)氨基]丙醛
3-[(苄氧基羰基)氨基]丙醛
3-[(BENZYLOXYCARBONYL)AMINO]PROPIONALDEHYDE
N-Phenylmethoxycarbonyl-3-aminopropanol
N-Phenylmethoxycarbonyl-3-Aminopropanal
3-((BENZYLOXYCARBONYL)AMINO)-1-PROPANOL&
3-[(Benzyloxycarbonyl)amino]propionaldehyde, Benzyl (3-oxopropyl)carbamate
3-Aminopropanal, N-CBZ protected
3-[(Benzyloxycarbonyl)amino]-1-propanal, 3-[(Carbobenzyloxy)amino]propionaldehyde
3-Hydroxypropyl(benzyloxycarbonyl)amine
3-Hydroxypropylcarbamic acid benzyl ester
物理化学性质
制备方法
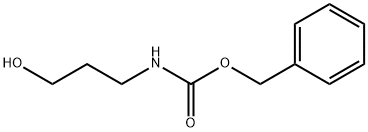
34637-22-4

65564-05-8
以3-(苄氧羰基氨基)-1-丙醇为原料合成N-苄氧羰基-3-氨基丙醛的一般步骤如下:将PCC(8.75g,40.6mmol,1.7当量)和Celite?(9g)在二氯甲烷(50mL)中搅拌5分钟,随后加入溶解于二氯甲烷(30mL)中的3-(苄氧羰基氨基)-1-丙醇(5.0g,24mmol)。反应4小时后,用乙醚(100mL)稀释反应混合物,并通过短的硅胶垫和Celite?过滤。将残留固体悬浮于二氯甲烷(25mL)中,并用乙醚(50mL)沉淀,使该混合物通过相同的过滤垫。此过程重复三次后,减压蒸发滤液,得到黄色油状物(5.0g)。通过柱层析纯化(40%乙酸乙酯的石油醚溶液),得到N-苄氧羰基-3-氨基丙醛,为无色粘性油状物(3.0g,14.5mmol),收率60%。Rf值为0.4(50%乙酸乙酯的石油醚溶液,PMA显色);1H NMR (CDCl3) δ: 2.75 (2H, t, J=5.7Hz, CH2), 3.49 (2H, 表观q, J=6.0Hz, CH2), 5.09 (2H, s, CH2), 5.15 (1H, br s, NH), 7.30-7.39 (5H, m, Ar), 9.81 (1H, s, CHO); 13C NMR (CDCl3) δ: 34.6, 44.2, 66.9, 128.2, 128.3, 128.7, 136.5, 165.4, 201.3; IR (neat) νmax: 3445, 1704, 1645 cm-1。
参考文献:
[1] Tetrahedron Letters, 2001, vol. 42, # 2, p. 183 - 185
[2] European Journal of Organic Chemistry, 2012, # 17, p. 3270 - 3277
[3] Tetrahedron, 1997, vol. 53, # 37, p. 12391 - 12404
[4] Tetrahedron Letters, 1987, vol. 28, # 33, p. 3827 - 3830
[5] Synlett, 2017, vol. 28, # 13, p. 1554 - 1557
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/09/19 | 42772 | 3-[(苯甲氧基羰基)氨基]丙醛 3-[(Benzyloxycarbonyl)amino]propionaldehyde, 95%, Thermo Scientific Chemicals | 65564-05-8 | 1g | 875元 |
| 2024/04/30 | C42772 | 3-[(苯甲氧基羰基)氨基]丙醛 3-[(Benzyloxycarbonyl)amino]propionaldehyde, 95% | 65564-05-8 | 1g | 1094元 |
| 2020/09/30 | C42772 | N-苄氧羰基-3-氨基丙醛 3-[(Benzyloxycarbonyl)amino]propionaldehyde95% | 65564-05-8 | 1g | 960元 |