7785-26-4
中文名称
左旋-alpha-蒎烯
英文名称
(1S)-(-)-alpha-Pinene
CAS
7785-26-4
EINECS 编号
232-077-3
分子式
C10H16
MDL 编号
MFCD00064145
分子量
136.23
MOL 文件
7785-26-4.mol
更新日期
2026/02/22 12:11:41
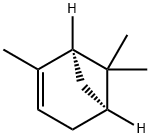 7785-26-4 结构式
7785-26-4 结构式
基本信息
中文别名
左旋-alpha-蒎烯(1S)-(-)-α蒎烯
(1S)-2,6,6-三甲基-二环[3.1.1]庚-2-烯
蒎烯
(1S)-(-)-α-蒎烯
(-)-Α-蒎烯,98%,含有数量不等的异构体
英文别名
(1s)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene(1S)-(-)-a-pinene
(1S)-(-)-pin-2-ene
(1S)-(-)-α-pinene
(1S)-2,6,6-trimethylbicyclo[3.1.1]-2-heptene
(1S,5S)-(-)-α-pinene
(S)-(-)-α-pinene
1S-(-)-a-pinene
2,6,6-trimethyl-,(1S)-Bicyclo[3.1.1]hept-2-ene
2-pinene,(1S,5S)-(-)-
6,6-trimethyl-(1s)-bicyclo[3.1.1]hept-2-en
Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl-, (1S)-
bicyclo[3.1.1]hept-2-ene,2,6,6-trimethyl-,(1S)-
l-α-pinene
α-pinene,(1S)-(-)-
(1S)-(-)-ALPHA-PINENE 99+% (87+% EE/GL&
Pinene,96%
(1S)-(-)-ALPHA-PINENE, 99+% (87+% E.E.)
(1S)-(-)-ALPHA-PINENE, 98% (81+% E.E.)
(1S)-(-)-ALPHA-PINENE 98+% FCC
所属类别
生物化工:提取物物理化学性质
外观性质无色透明液体,有松节油的气味,不溶于水,溶于无水乙醇、乙醚、氯仿等多数有机溶剂。闪点:33℃熔点:-64℃沸点:156℃折光率:1.466密度:0.858[α]20D -45.5 ° (C=neat)
熔点-64 °C(lit.)
比旋光度-41.5 º (c=neat)
沸点156-158 °C(lit.)
密度0.874 g/mL at 25 °C
蒸气密度4.7 (vs air)
蒸气压~3 mm Hg ( 20 °C)
FEMA2902
折射率n20/D 1.466
闪点90 °F
储存条件2-8°C
溶解度氯仿(微溶)、甲醇(微溶)
形态液体
颜色透明无色
气味 (Odor)at 10.00 % in dipropylene glycol. sharp warm resinous fresh pine
爆炸极限值(explosive limit)0.8%(V)
生物来源Pinus spp.
香型herbal
旋光度 (Optical Rotation)[α]22/D 42°, neat
水溶解性INSOLUBLE
JECFA Number1329
Merck14,7445
检测方法GC
BRN1903790
主要应用flavors and fragrances
化妆品成分功效PERFUMING
InChI1S/C10H16/c1-7-4-5-8-6-9(7)10(8,2)3/h4,8-9H,5-6H2,1-3H3/t8-,9-/m0/s1
InChIKeyGRWFGVWFFZKLTI-UHFFFAOYSA-N
SMILES[C@@H]21C([C@@H](C2)C(=CC1)C)(C)C
LogP4.48 at 37℃
EPA化学物质信息2-Pinene, (1S,5S)-(-)- (7785-26-4)
安全数据
知名试剂公司产品信息
Acros Organics
(1S)-(-)-alpha-蒎烯(1S)-(-)-alpha-Pinene, 98%(7785-26-4)
Alfa Aesar
(-)-α-蒎烯,98%,含有数量不等的异构体(-)-alpha-Pinene, 98%, cont. variable amounts of enantiomer(7785-26-4)
Sigma Aldrich
7785-26-4(sigmaaldrich)左旋-alpha-蒎烯价格(试剂级)
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | P0440 | (1S)-(-)-α-蒎烯 (1S)-(-)-alpha-Pinene | 7785-26-4 | 25ML | 150元 |
| 2025/12/22 | HY-N0549 | 左旋-alpha-蒎烯 (-)-α-Pinene | 7785-26-4 | 5G | 500元 |
| 2025/12/22 | P0440 | 左旋-alpha-蒎烯 (1S)-(-)-α-Pinene | 7785-26-4 | 100ML | 260元 |
常见问题列表
生物活性
(-)-α-Pinene 是一种单萜类化合物,通过在 BZD 结合位点作为部分调节剂与 GABAA-苯二氮卓 (BZD) 受体直接结合,显示出增强睡眠的特性。靶点
|
Human Endogenous Metabolite
|
体外研究
(-)-α-pinene enhances the quantity of non-rapid eye movement sleep (NREMS) without affecting the intensity of NREMS by prolonging GABAergic synaptic transmission, acting as a partial modulator of GABAA-BZD receptors and directly binding to the BZD binding site of GABAA receptor.



