Silbercarbonat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R20:Gesundheitsschädlich beim Einatmen.
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Chemische Eigenschaften
Silver carbonate (chemical formula: Ag2CO3) is a light yellow or yellow-green powder, slightly soluble in water, and easily turns dark purple or dark gray under long-term light. After heating, it will decompose to produce silver oxide, which is used to prepare other silver compounds.

Verwenden
Silver carbonate is a mild oxidizing agent for conversion of alcohols to aldehydes and ketones. It is also used as Biological stain and Koenigs-Knorr glycosylation.
Application
Silver Carbonate on Celite is a reagent used in the synthesis of 3-Oxo-12a-hydroxy-5β-cholanoic Acid which is a keto bile acid derivative.
synthetische
Silver carbonate is prepared by the reaction of sodium carbonate and silver nitrate.
Reaction: Dissolve 53g of sodium carbonate in 600ml of water and slowly add it to a solution of 172g of silver nitrate dissolved in 2L of water (10min). Silver nitrate is in a slight excess. The reaction mixture was vigorously stirred with a mechanical stirrer, and the silver carbonate was filtered off, washed with a small amount of acetone to facilitate drying, and then air-dried. All operations must be carried out in a dark room.
Reaktionen
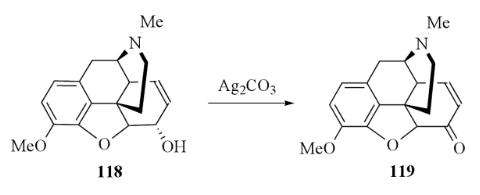
Silver carbonate is not a powerful oxidizing agent but it is useful in Organic chemistry. Rapoport et al. were probably the first to use silver carbonate for the oxidation of alcohols to carbonyl derivatives. Rapoport refluxed codeine with silver carbonate in benzene and obtained a 75% yield of codeinone. In later work King et al. oxidized codeine with silver carbonate in refluxing toluene or xylene and obtained an 85% yield of codeinone with a much shorter reaction time.
Allgemeine Beschreibung
Odorless yellow to brown solid. Sinks in water.
Reaktivität anzeigen
Silver carbonate is a carbonates salt that has weak oxidizing or reducing powers. It is decomposed by acids with the evolution of carbon dioxide.
Health Hazard
Contact with eyes causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of the skin (argyria).
Brandgefahr
Behavior in Fire: Decomposes to silver oxide, silver, and carbon dioxide; the reaction is not hazardous.
Silbercarbonat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

