Caesiumcarbonat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R68:Irreversibler Schaden möglich.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-Sätze Betriebsanweisung:
S22:Staub nicht einatmen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S27:Beschmutzte, getränkte Kleidung sofort ausziehen.
Beschreibung
Cesium carbonate is a basic carbonate and is used in organic synthesis as a mild inorganic base. It can be used in C, N, O alkylation and arylation reactions. Cesium carbonate not only promotes the carbonylation of alcohols and carbamination of amines but also inhibits side reactions common in other processes. It is also used in six-membered annulation, intramolecular and intermolecular cyclizations, Suzuki coupling, aza-Henry, nucleophilic substitution, cross-coupling and different cycloaddition reactions.
Chemische Eigenschaften
Colourless crystals or white powder , easily soluble in water, quickly absorbs moisture when placed in the air. The aqueous solution of cesium carbonate is strongly alkaline and can react with acid to produce the corresponding cesium salt and water, and release carbon dioxide.
Verwenden
Cesium carbonate is a versatile reagent for organic synthesis. This inorganic base has been employed in numerous organic applications ranging from N-protection of amino acids to a base in either the Horner- Wadsworth-Emmons reaction or in Suzuki couplings. It has also found use as a catalyst for ethylene oxide polymerization, in coating for spatter-free welding of steel in CO2, as a functional interlayer in photovoltaic devices and in oxide cathodes. Cesium carbonate is also used in the beer-brewing industry to make the "head" of beer foamier.
Application
Cesium carbonate is widely utilized as a precursor for other cesium compounds. It acts as a base in sensitive organic reactions. It can be used as a base in C-C and C-N cross-coupling reactions such as Suzuki?Miyaura, Heck, and Buchwald-Hartwig amination reactions. It finds use in solar cells as it increases the power conversion efficiency of cells through the transfer of electrons. It is also used in the production of special optical glasses, petroleum catalytic additives, special ceramics and in the sulfuric acid industry. It is useful in the N-alkylation (of sulfonamides, beta-lactams, indoles, heterocycles and several sensitive nitrogen compounds), carbamination of amines, carbonylation of alcohols and aerobic oxidation of alcohols into carbonyl compounds without polymeric by products. Promotes the efficient O-alkylation of alcohols to form mixed alkyl carbonates.
synthetische
cesium carbonate can be prepared by thermal decomposition of caesium oxalate. Upon heating, caesium oxalate is converted to caesium carbonate with emission of carbon monoxide.
Cs2C2O4 → Cs2CO3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.
2 CsOH + CO2 → Cs2CO3 + H2O
Reaktionen
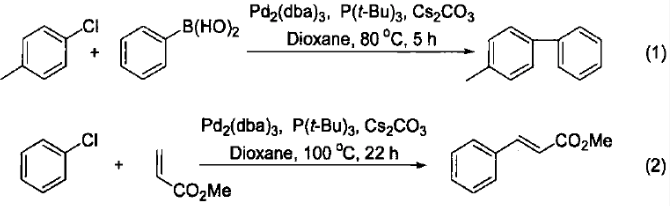
The numerous properties of caesium carbonate in organic synthesis stem from the relatively weak Lewis acidity of the caesium ion, enabling its solubility in organic solvents such as alcohols, DMF, and diethyl ether. Caesium carbonate serves as an effective inorganic base in numerous palladium-catalysed reactions, including the Heck, Suzuki, and Sonogashira reactions. As shown in Equation 1 [1], the Suzuki cross-coupling reaction achieves an 86% yield when facilitated by caesium carbonate. However, the same reaction yields only 29% and 50% when using sodium carbonate or triethylamine, respectively. In the Heck reaction between methacrylates and chlorobenzene, caesium carbonate demonstrates markedly superior performance compared to other inorganic bases (e.g., potassium carbonate, sodium acetate, triethylamine, potassium phosphate) (Equation 2 [2]).
Allgemeine Beschreibung
Cesium carbonate is a powerful inorganic base widely used in organic synthesis. It is a potential chemoselective catalyst for the reduction of aldehydes and ketones to alcohols. It is employed as base for the Heck coupling reaction of 4-trifluoromethyl-1-chlorobenzene and aryl chlorides.
Hazard
Cesium carbonate is a danger compound.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H361: Suspected of damaging fertility or the unborn child
H373: May cause damage to organs through prolonged or repeated exposure
Sicherheitsprofil
Moderately toxic by
ingestion. Mutation data reported. When
heated to decomposition it emits acrid
smoke and fumes. See also CESIUM.
läuterung methode
Crystallise it from ethanol (10mL/g) by partial evaporation. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 988 1963.]
Caesiumcarbonat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

