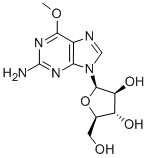
Nelarabine
- CAS No.
- 121032-29-9
- Chemical Name:
- Nelarabine
- Synonyms
- MAY;Arranon;Nelzarabine;506u;506U78;CS-1953;GW 506U78;NELARABIN;NELARABINE;Nelarabine D4
- CBNumber:
- CB01011878
- Molecular Formula:
- C11H15N5O5
- Molecular Weight:
- 297.27
- MDL Number:
- MFCD00871078
- MOL File:
- 121032-29-9.mol
- MSDS File:
- SDS
| Melting point | 209-217° |
|---|---|
| alpha | D20 +55.9° (c = 0.27 in DMF) |
| Boiling point | 721.0±70.0 °C(Predicted) |
| Density | 1.98±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated), Water (Slightly, Sonicated) |
| form | Solid |
| pka | 13.07±0.70(Predicted) |
| color | White |
| CAS DataBase Reference | 121032-29-9(CAS DataBase Reference) |
| FDA UNII | 60158CV180 |
| NCI Dictionary of Cancer Terms | 506U78; Arranon; nelarabine |
| NCI Drug Dictionary | Arranon |
| ATC code | L01BB07 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
Nelarabine price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | N1144 | Nelarabine | 121032-29-9 | 100MG | $669 | 2023-01-07 | Buy |
| TCI Chemical | N1144 | Nelarabine | 121032-29-9 | 25MG | $214 | 2021-12-16 | Buy |
| Cayman Chemical | 20248 | Nelarabine ≥98% | 121032-29-9 | 1mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 20248 | Nelarabine ≥98% | 121032-29-9 | 5mg | $161 | 2024-03-01 | Buy |
| Cayman Chemical | 20248 | Nelarabine ≥98% | 121032-29-9 | 10mg | $283 | 2024-03-01 | Buy |
Nelarabine Chemical Properties,Uses,Production
Physical properties
White or almost white crystalline powder. This product is slightly soluble in water, the solubility in water is about 8-9mg/ml (25 ℃, PH = 4~10).
Developed by GlaxoSmithKline
Nelarabine is a prodrug for deoxyguanosine analogue ——9-beta-D-arabinofuranosyl guanine (ara-G). Nelarabine demethylates in the role of adenosine deaminase (ADA) and turns into ara-G. Then, It turns into active ara-G triphosphate (ara-GTP) in the role of the deoxyguanosine kinase and deoxycytidine kinase and under the action of the monophosphorylation pathway. Ara-GTP can accumulate in leukemia cells, and bind to DNA to inhibit DNA synthesis, and accelerate leukemia cell death. In addition, the anti-cancer mechanism of Nelarabine may also be related to its cytotoxicity and somatic toxicity.
Nelarabine was first successfully developed by GlaxoSmithKline. On October 28, 2005 under the approval of the US Food and Drug Administration, it became a new drug for curing T-cell acute lymphoblastic leukemia (T-ALL) and T-Cells lymphoblasticlymphoma (T-LBL) which are resistant to at least two types of chemotherapy regimens or relapsed after initial treatment. This new drug officially listed in the United States in 2006. This product didn’t apply for patent and administrative protection in China, so the development of this product does not exist intellectual property issues.
Pharmacology and pharmacokinetics
Nelarabine is a prodrug for deoxyguanosine analogue 9-β-arabinosyl guanine (ara-G). Ara-G has poor water solubility and can become Nelarabine with methoxy group in vivo. It was mono-phosphorylated into 5'-triphosphate ara-G, when catalyzed by adenosine deaminase and deoxycytidine kinase. Ara cells-GTP is accumulated in leukemia. PNP deficiency leads to the selective accumulation of dGTP in T cells, whereas dGTP rapidly degrades in B cells without accumulation in B cells. Intracellular accumulation of dGTP can inhibit DNA synthesis. Arabinosyl guanine can selectively kill cells with T cell leukemia, leading to cell death. Other mechanisms may be cytotoxicity and systemic toxicity of Nelarabine.
Recurrent pharmacokinetic study of leukemia or lymphoma in patients found that Nelarabine and ara-G can eliminated in plasma quickly. the two half-life were 30 minutes and 3 hour when giving 1500mg/m2 dose of Nelarabine. The study also found that ara-G usually reached the peak concentration at the end of Nelarabine administration, and its peak concentration value is usually greater than the peak concentration of Nelarabine, indicating that Nelarabine in the body can be quickly and completely transformed into ara-G. Nelarabine and ara-G were partially abolished by the kidneys, and the mean renal excretion rates of Nelarabine and ara-G measured 24 hours after administration of Nelarabine in 28 adult patients were 6.6 ± 4.7% and 27 ± 15% .
Clinical trials
A large amount of clinical trials have shown that results of Nelarabine’s treatment of T-cell lymphocytic leukemia (T-PLL) and peripheral T-cell lymphoma are encouraging.
The potential indications for Nelarabine also include non-T-cell disease with high levels of nucleotide kinases in the target tissue. In fact, phase I and II clinical trials have shown that Nelarabine alone or in combination with other drugs also have a certain effect on the treatment of B-cell chronic lymphocytic leukemia.
Intracellular levels of ara-GTP can predict whether patients respond to Nelarabine. And the combination of Nelarabine and other drugs can be used to further expand the use of the product range.
In addition, this drugs can be improved in the administration method. Now the administration method is 1-2 hours intravenous infusion. Patients receive Nelarabine daily for 5 days or Nelarabine was administered on days 1, 3, and 5. Finally, since the product selectively acts on T-cells without the bone marrow toxicity of headache, the development of myelosuppression can be exploited.
At present, there are three institutes declare the right of raw materials and preparation of Nelarabine: Beijing Fu Ruikang Pharmaceutical Technology Research Institute, Hangzhou Rong Li Pharmaceutical Technology Co., Ltd., Jiangsu Aokang medicine.
Usage and dosage
Nelarabine is mainly used for patients who have received at least two chemotherapy regimens but still have no response, or have occurred recurrent acute T-cell lymphoblastic leukemia and T-cell lymphoblastic lymphoma.
Adult patients received nelarabine i.v. over 2 hours on days 1, 3 and 5 in every 21 days with the recommendation dose of 1500mg/m2 in every 21 days and without dilution; pediatric patients received this regimen over 1 hour for 5 consecutive days with the recommendation dose of 650mg/m2 in every 21 days, without dilution. The recommended treatment period for adults and children is unspecified. In clinical trials, unless the patient get worse and cannot tolerate toxicity. They should treated with bone marrow transplant immediately or no longer be treated.
Note
Nelarabine-induced neurotoxicity is a dose-limiting toxic reaction. Patients should pay close attention during the medication whether there is confusion, drowsiness, convulsions, ataxia, paresthesia and hypoesthesia and other symptoms. Patients who had undergone intrathecal chemotherapy or cranial radiotherapy had an increased risk of neurotoxic response after Nelarabine administration.
Nelarabine may also cause leukopenia, thrombocytopenia, anemia and neutropenia (including febrile neutropenia), so the application of Nelarabine should be routine for patients with complete blood count (including platelet count).According to standard treatment regimen, Nelarabine used in patients with tumor lysis syndrome complicated by hyperuricemia, can be relieved by intravenous hydration; in the presence of hyperuricemia risk patients may also consider taking allopurinol for treatment. Immunodeficient patients receiving Nelarabine should avoid live vaccines.
Description
Nelarabine is a new member of the purine nucleoside antimetabolite class of drugs. It was launched as an intravenous infusion for treating relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) after at least two prior chemotherapy regimens. Nelarabine is a pro-drug of 9-b-D-arabinofuranosylguanine (ara-G), a deoxyguanosine derivative with a high level of T-cell selective cytotoxicity. Although Ara-G has been known since the 1960s, it has not been used in clinical studies due to its poor solubility. Nelarabine is the O-methyl derivative of ara-G with approximately 10 times greater aqueous solubility. It is demethylated in vivo by adenosine deaminase to produce ara-G, which is subsequently converted to the active 5’-triphosphate, ara-GTP. Accumulation of ara-GTP in leukemic blasts allows for incorporation into DNA, leading to inhibition of DNA synthesis and cell death.
Originator
Glaxo Wellcome (US)
Uses
A chemotherapy drug used in the treatment of relapsed/refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL).
Definition
ChEBI: Nelarabine is a purine nucleoside in which O-methylguanine is attached to arabinofuranose via a beta-N(9)-glycosidic bond. Inhibits DNA synthesis and causes cell death; a prodrug of 9-beta-D-arabinofuranosylguanine (ara-G). It has a role as an antineoplastic agent, a DNA synthesis inhibitor and a prodrug. It is a purine nucleoside, a beta-D-arabinoside and a monosaccharide derivative. It is functionally related to a guanine and a 9-beta-D-arabinofuranosylguanine.
brand name
Arranon (SmithKline Beecham).
Clinical Use
Antineoplastic agent:
T-cell acute lymphoblastic leukaemia (T-ALL)
T-cell lymphoblastic lymphoma (T-LBL)
Synthesis
The drug was synthesized by enzymatic coupling of arabinosyluracil 87, prepared according to literature and 2-amino- 6-methoxy purine 88 using purine nucleoside phosphorylase (PNP) and uridine phosphorylase (UP) in phosphate buffer for 30 days to give the nelarabine (XIII) in 48% yield.
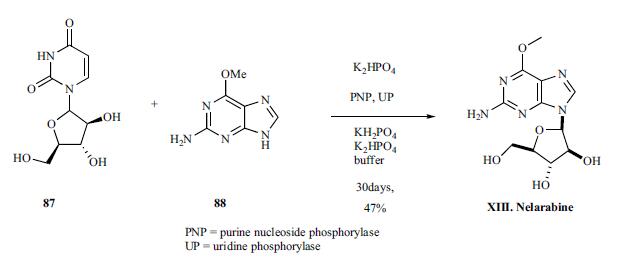
Metabolism
Nelarabine is a pro-drug of the deoxyguanosine analogue ara-G. Extensive metabolism by O-demethylation by adenosine deaminase to form ara-G, which undergoes hydrolysis to form guanine. In addition, some nelarabine is hydrolysed to form methylguanine, which is O-demethylated to form guanine. Guanine is N-deaminated to form xanthine, which is further oxidised to yield uric acid. Nelarabine and ara-G are partially eliminated by the kidneys.
storage
Store at +4°C
References
[1] cohen mh, johnson jr, massie t, et al. approval summary: nelarabine for the treatment of t-cell lymphoblastic leukemia/lymphoma. clin cancer res. september 2006. 18:5329–35.
[2] yesid alvarado, mary alma welch, ronan swords, john bruzzi, ellen schlette, francis j. giles. nelarabine activity in acute biphenotypic leukemia. leukemia research. november 2007. 31(11): 1600-1603.
Nelarabine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Nanjing Baifuli Technology Co., Ltd. | +86-15335185688 | sales@unisyn.cn | CHINA | 332 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
View Lastest Price from Nelarabine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2022-09-24 | Nelarabine
121032-29-9
|
US $0.00-0.00 / kg | 1kg | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2022-08-17 | Nelarabine
121032-29-9
|
US $20.00 / kg | 1kg | 99% | 5000 Ton | Hebei Duling International Trade Co. LTD | |
 |
2021-09-29 | Nelarabine
121032-29-9
|
US $1.00 / g | 1g | 99.0% | 5kg/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Nelarabine
121032-29-9
- US $0.00-0.00 / kg
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- Nelarabine
121032-29-9
- US $20.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Nelarabine
121032-29-9
- US $1.00 / g
- 99.0%
- WUHAN FORTUNA CHEMICAL CO., LTD