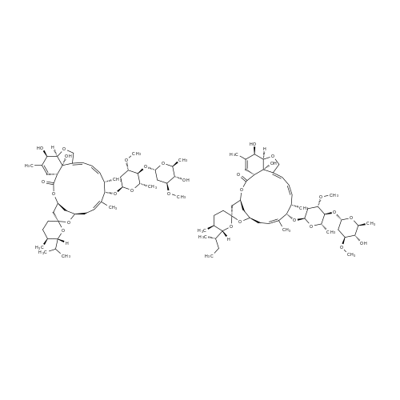
Ivermectin
- CAS No.
- 70288-86-7
- Chemical Name:
- Ivermectin
- Synonyms
- IVERMECTINE;IVOMEC;IVERMECTIN HCL;VERMECTIN;Stromectol;Ivermectin EP2000;Avermectin B1a Dihydro Analog;mk933;Uvemec;Vermic
- CBNumber:
- CB0415655
- Molecular Formula:
- C48H74O14
- Molecular Weight:
- 875.09
- MDL Number:
- MFCD00869511
- MOL File:
- 70288-86-7.mol
- MSDS File:
- SDS
| alpha | D +71.5 ± 3° (c = 0.755 in chloroform) |
|---|---|
| RTECS | IH7891500 |
| storage temp. | 2-8°C |
| solubility | H2O: ≤1.0% KF |
| form | powder |
| color | White |
| Water Solubility | 4mg/L(temperature not stated) |
| BCS Class | 4/3 |
| Stability | Stable for 2 years as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChIKey | AZSNMRSAGSSBNP-XPNPUAGNSA-N |
| CAS DataBase Reference | 70288-86-7(CAS DataBase Reference) |
| FDA 21 CFR | 556.344; 558.300; 558.4 |
| EWG's Food Scores | 1 |
| FDA UNII | 8883YP2R6D |
| NCI Drug Dictionary | ivermectin |
| ATC code | D11AX22,P02CF01 |
| EPA Substance Registry System | Ivermectin (70288-86-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H311-H410 | |||||||||
| Precautionary statements | P264-P270-P273-P280-P301+P310-P302+P352+P312 | |||||||||
| Hazard Codes | T,Xn,T+ | |||||||||
| Risk Statements | 61-25-36-36/38-22-28 | |||||||||
| Safety Statements | 53-26-45-36-36/37-28 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29322090 | |||||||||
| Toxicity | LD50 in dogs, rhesus monkeys (mg/kg): about 80, more than 24 orally. (MSDS Merck & Co. Inc., 2003) | |||||||||
| NFPA 704 |
|
Ivermectin price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | I8898 | Ivermectin | 70288-86-7 | 1g | $110 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1354309 | Ivermectin United States Pharmacopeia (USP) Reference Standard | 70288-86-7 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP864 | Ivermectin British Pharmacopoeia (BP) Reference Standard | 70288-86-7 | 250MG | $252 | 2023-06-20 | Buy |
| Alfa Aesar | J62777 | Ivermectin | 70288-86-7 | 250mg | $29.8 | 2024-03-01 | Buy |
| Alfa Aesar | J62777 | Ivermectin | 70288-86-7 | 1g | $83.7 | 2024-03-01 | Buy |
Ivermectin Chemical Properties,Uses,Production
Ivermectin poisoning
Ivermectin also called Ivomec, is a kind of medicine that has a good effect on mite disease treatment.It will cause poisoning if were overcommit ted. The symptoms of ivermectin poisoning are as follows: vomiting, accelerated breathing ,weakness and paralysis in arms and legs, and congestive heart failure that would cause death.
Rescue : Just take orally mung bean licorice drink detoxification with 10% of glucose and inject dexamethasone when necessary.
Effects of ivermectin
Ivermectin is white or light yellow crystalline powder and soluble in methyl alcohol, ester and aromatic hydrocarbon but water. Ivermectin is a kind of antibiotic medicine which has a driving and killing effect on nematodes, insects and mites. Injection and troche which are made from ivermectin are mainly used in the treatments of livestock’s gastrointestinal nematode, bovine hypodermosis, calf fly maggot, sheep nasal fly maggot, and scabies of sheep and pigs. Besides, ivermectin can also be available for the treatment of plant-parasitic nematodes(ascarid, lungworm) in poultry. In addition, it can also be made into agricultural insecticide to kill mite, plutella xylostella, cabbage caterpillar, leaf miner, phylloxera and nematode which are widely parasitic in plants. The most outstanding feature of this insecticide is that it has little side effects and can drive and kill many kinds of parasites both internally and externally at a time.
The above information is edited by the Chemicalbook Bai Linlin.
Botanical pesticide of ivermectin
Ivermectin is a kind of botanical pesticide which is made of cynanchum komarovii, sophora alopecuroides and many other plants and Chinese herbal pieces through grinding, dissolving, adding promoters and penetrant and processing the mixtures. Its action mechanism is mainly based on contact poisoning with stomach toxicity as an complementary, which has a promoting effect on plant growth. It can be used to prevent and kill all kinds of aphids and defoliators. If diluted with water to 1000~2000 times and spayed, its control effect can reach above 98%. Ivermectin is a new generation of botanical pesticide that is low toxicity, low residual and has no hazard to people, livestock and environment.
The insecticidal mechanism of ivermectin is to inhibit the synthesis of chitin of insect epicutile. The main component of chlorbenzuron is stomach poison, but it can also intrude into insect epicutile and take effect. The prevention and treatment of defoliator has many advantages such as special action mechanism, good effect, long residual period, low cost, tolerant to rain shower, not easy to generate insecticide resistance and safe for plants, human, livestock and environment.
Instruction of ivermectin
[Pharmacologic action] Ivermectin has killing effects on microfilaria, but its exact mechanism is not clear. It can be used as agonist for neurotransmitter γ-tyrosine(GABA) to break the transfer process of synapses in the central nervous system neurons mediated by GABA, which can lead to death of polypide by paralyzing its nervous system. As for adult insects, ivermectin doesn’t work, but it can affect the normal development of microfilariae of onchocerca volvulus in the female uterus, and can inhibit the release from the pregnant worm palace. Ivermectin has slow and persistent effect on microfilaria compared with diethylcarbamazine. It can quickly reduce the number of microfilariae of patients with the skin. However, it has a relatively slow effect on the larva of patients’ cornea and anterior chamber of eyes, therefore the number of larva in this area declines rather slow.
[Pharmacokinetics]After taking the medicine orally, blood concentration will reach the peak in 4 hours and T1/2 in 10 hours. Animal experiments showed only l~2% of oral doses appears in the urine in the form of original drug and the others is excreted with excrement. The drug concentration in the liver and adipose is very high, but cannot penetrate the blood brain barrier, while in the nerve cells including GABA the concentration is rather low, hence we rarely see the response of central nervous system after taking medicine.
[Adaptation disease]the main medicine of treating onchocercosis.
[Usage and dosage] Take the medicine orally one hour before the meal. The usual dose of onchocercosis treatment: 0.15-0.2mg/kg according to the weight, every 6-12 month at a time, depending on the symptoms and the reappear time of microfilaria. It can prevent the further development of ocular disorders(mostly caused by microfilaria), but this can’t be a radical treatment, because it’s inability to kill adult insects.
[Preparation and specification] Ivermectin tablet 6mg
[Untoward effects] Non-infected people and other Mammalian species are well tolerant towards this product. Symptoms like drowsiness, ataxia, mydriasis, tremble and even death will appear when given mega dose of this product to animals. The patients who suffer from onchocercosis may have transience and slight side effect after taking this medicine, most of which are rash and pruritus(caused by the death of microfilaria in the skin), lymphadenopathy(gall, seen in the neck, armpit, and inguen). It is rare to see the symptoms such as swirl, postural hypotension(faint), radiation, headache, joint sore, weakness and so on. There is not much change in ocular lesions. Occasionally, we can see the change of electrocardiogram but the significance is unclear. This product has no carcinogenic and teratogenic effect.
Chemical properties
Ivermectin contains at least 80% of 22,23-dihydroavermectin B1a and less than 20%22,23-dihydroavermectin B1b. It is white powder. [α]D+71.5°±3°(C=0.755,chloroform). Maximum absorption(methyl alcohol) of UV is 238,245 nm(ε27100,30100). The water solubility is about 4μg/ml and it is easily soluble in methyl ethyl ketone, propylene glycol or polypropylene glycol, but not in saturated hydrocarbon, for example cyclohexane.
B1a:[71827-03-7]. Ethyl alcohol-water crystallization, melting point: 155~157℃.
Uses
1. Ivermectin is available for controlling and treating onchocerca volvulus, and its untoward effect is lower than that of carbamazine.
2. Ivermectin can be used as a kind of antiphrastic drug and has an anthelmintic activity towards nematode, hookworm, roundworm, worm, insect and mite.
Production methods
Under normal pressure and at room temperature, ivermectin can be obtained through the corresponding compounds (I) in benzene hydrogenated and catalyzed by the three (triphenylphosphine) rhodium chloride.
Pharmacology and mechanism of action
Ivermectin belongs to a class of substances known as the avermectines. These are macrocylic lactones produced by fermentation of an actinomycete, Streptomyces avermitilis. Ivermectin is a broad spectrum agent active against nematodes and arthropods in domestic animals and is thus widely used in veterinary medicine [1]. The drug was first introduced in man in 1981. It has been shown to be effective against a wide range of nematodes such as Strongyloides sp., Trichuris trichiura, Enterobius vermicularis, Ascaris lumbricoides, hook worms and Wuchereria bancrofti. However, it has no effect against liver flukes and cestodes [2].
Presently it is regarded as the drug of choice in onchocerciasis. It is a potent microfilaricide, but it does not possess any significant macrofilaricidal effect[3]. Between 2 to 3 days afteroral administration, microfilariae in the skin start to disappear rapidly, while those in the cornea and the anterior chamber of the eyes are eliminated more gradually. This is an effect which lasts for up to 12 months [4–5]. One month after administration, the microfilariae in the uterus of the worms are also affected where they get trapped and eventually degenerate and get resorbed[5]. This long-term suppression of microfilariae has potential usefulness in interrupting the transmission of the disease [6,7]. The mechanism of action of ivermectin against onchocerciasis is not clearly understood, but it is presumed to be a GABA-agonist. In susceptible organisms the drug acts by potentiating the release of gamma-aminobutyric acid (GABA) at postsynaptic sites on the neuromuscular junction rendering the nematode paralysed [8].
Indications
Ivermectin is the drug of choice against onchocerciasis. It is, however, an expensive drug and its distribution is still restricted. The role of ivermectin in lymphatic filariasis is not yet well investigated.
Contraindications and precautions
Experience of the drug is lacking for children under 5 years of age. Since the drug acts by potentiating GABA, there is a concern that CNS effects may be seen in humans whose bloodbrain barrier is impaired (e.g. by meningitis, trypanosomiasis). Ivermectin does not cross the blood-brain barrier; however, severe CNS toxicity has been reported from animals without a blood-brain barrier (e.g. collie dog) . The relevance of this in humans is not known.
Side effects
About 1.5 million people, mainly in West Africa, have now been treated with ivermectin, and all the evidence indicates that it is a safe drug, which is suitable for large scale treatment programmes[9,10, 11]. Side effects reported include fever, itching, dizziness, oedema, mild Mazzotti reaction, and minimal ocular inflammation in patients with eye involvement. The side effects usually occur during the first 3 days after treatment and are dose dependent. The reported incidences of side effects vary. In one review [11], which covered 50,929 patients who had been treated with ivermectin, around 9% were reported to have suffered from side effects. The most frequent reaction was symptomatic postural hypotension. The authors reported that the incidence of side effects was directly proportional to the microfilarial density in the skin[11]. In hyperendemic areas, a much higher incidence of adverse reactions may be seen [12]. Homeida et al. [13], reported a high incidence of prolongation of the prothrombin time in Sudanese patients treated with ivermectin, but this has not been confirmed in other studies
[14–16].
Interactions
There are no reports of harmful drug interactions, but theoretically, the drug may potentiate the effects of other drugs that are agonists of the GABA receptors (e.g. benzodiazepines and sodium valproate).
Preparations
Mectizan® (Merck Sharp & Dohme). Tablets 6 mg.
Reference
1. Campbell WC, Fisher MH, Stapley EO, Albers-Schönberg G, Jacob TA (1983). Ivermectin: apotent new antiparasitic agent. Science, 212, 823–828.
2. Campbell WC (1991). Ivermectin as an antiparasitic agent for use in humans. Annu Rev Microbiol,45, 445–474.
3. Goa KL, McTavish D, Clissold SP (1991). Ivermectin: A review of its antifilarial activity,pharmacokinetic properties and clinical trials in onchocerciasis. Drugs, 42, 640–658.
4. Awadzi K, Dadzie KY, Schultz-Key H, Haddock DRW, Gilles HM, Aziz MA (1984). Ivermectinin onchocerciasis. Lancet, 2, 291.
5. Schultz-Key H (1990). Observations on the reproductive biology of Onchocerca volvulus. ActaLeiden, 59, 27–43.
6. Taylor HR, Pacqué M, Munoz B, Greene BM (1990). Impact of mass treatment of onchocerciasiswith ivermectin on the transmission of infection. Science, 250, 116–118.
7. Cupp EW, Bernardo MJ, Kiszewski AE, Collins RC, Taylor HR, Aziz MA, Greene BM (1986).The effects of ivermectin on transmission of Onchocerca volvulus. Science, 231, 740–742.
8. Pong S-S, Wang CC, Fritz LC (1980). Studies on the mechanism of action of avermectin Bla: stimulationof release of gamma-aminobutyric acid from brain synaptosomes. J Neurochem, 34, 351–358.
9. Pacque M, Munoz B, Poetschke G, Foose J, Greene B, Taylor H (1990). Pregnancy outcome afterinadvertent ivermectin treatment during community-based distribution. Lancet, 336, 1486–1489.
10. Whitworth JAG (1992). Drug of the month: ivermectin. Tropical doctor, 22, 163–164.
11. De Sole G, Remme J, Awadzi K, Accorsi S, Alley ES, Ba O, Dadzie KY, Giese J, Karam M,Keita FM (1989). Adverse reactions after large-scale treatment of onchocerciasis with ivermectin:combined study from eight community trials. Bull WHO, 67, 707–719.
12. Whitworth JAG, Morgan D, Maude GH, Taylor DW (1988). Community-based treatment withivermectin. Lancet, 2 97–98.
13. Homeida MM, Bagi IA, Ghalib HW, el Sheikh HE, Ismail A, Yousif MA, Sulieman S, Ali HM,Bennett JL, Williams J (1988). Prolongation of prothrombin time with ivermectin. Lancet, i,1346–1347.
14. Pacque MC, Munoz B, White AT, Williams PN, Greene BM, Taylor HR (1989). Ivermectin andprothrombin time. Lancet, 1, 1140.
15. Whitworth JAG, Hay CRM, McNicholas AM, Morgan D, Maude GH, Taylor DW (1992).Coagulation abnormalities and ivermectin. Ann Trop Med Parasitol, 86, 301–395.
16. Richards Jr FO, McNeely MB, Bryan RT, Eberhard ML, McNeely DF, Lamie PJ, Spencer HC(1989). Ivermectin and prothrombin time. Lancet, i, 1139–1140.
17. Campbell WC, Benz GW (1984). Ivermectin: a review of efficacy and safety. J Vet PharmacolTher, 7, 1–16.
Description
Ivermectin is an antiparasitic agent effective in the treatment of onchocerciasis, or "river blindness". Since ivermectin acts to prevent the adult worm from producing microfilariae, it needs to be administered only once or twice a year.
Chemical Properties
Crystalline Solid
Originator
Merck (USA)
Uses
Semi-synthetic derivative of Abamectin; consists of a mixture of not less than 80% component B1a and not more than 20% component B1b. Anthelmintic (Onchocerca).
Uses
antiparasitic
Uses
beta-lactamase inhibitor; antibiotic
Uses
Positive allosteric modulator of α7 neuronal nicotinic acetylcholine receptor; also modulates glutamate-GABA-activated chloride channels.
Definition
ChEBI: LSM-5397 is a milbemycin.
Preparation
The synthesis of ivermectin involves the hydrogenation of the naturally occurring avermectin B1 (abamectin) at the double bond linking C-22 and C-23. This results in a mixture of two homologues, 22, 23-dihydroavermectin B1a (H2B1a) and 22, 23-dihydroavermectin B1b (H2 B1b). The a- and b- nomenclature refers to the presence on C-25, of either a secondary butyl side chain or an isopropyl group, respectively. The biological activities of H2B1a and H2B1b are similar. Large-scale separation of the two homologues is not practical, and, hence, ivermectin is marketed as a mixture of H2 B1a (>80%) and H2 B1b (<20%).
Indications
Ivermectin (Mectizan) acts on parasite-specific inhibitory glutamate-gated chloride channels that are phylogenetically related to vertebrate GABA-gated chloride channels. Ivermectin causes hyperpolarization of the parasite cell membrane and muscle paralysis.At higher doses it can potentiate GABA-gated chloride channels. It does not cross the blood-brain barrier and therefore has no paralytic action in mammals, since GABA-regulated transmission occurs only in the central nervous system (CNS). Ivermectin is administered by the oral and subcutaneous routes. It is rapidly absorbed. Most of the drug is excreted unaltered in the feces. The half-life is approximately 12 hours.
Manufacturing Process
Avermectin is produced by biotechnological methods with the aid of
Streptomyces avermitilis.
Preparation of Catalyst I
Rhodium trichloride trihydrate (1.00 g, 3.80 mmol) was dissolved in water
(5.0 ml) with heating (70°C). A solution of triphenylphosphine (1.95 g, 7.43
mmol) in acetone (25.0 ml) was then added under a nitrogen atmosphere in
the course of 20 min. After 10 min hydrazine hydrate (1.90 ml; 39.09 mmol)
was added with stirring and the mixture was heated at reflux temperature for
3 hours, then kept at 45°C for a further 1 hour. The crystalline solid was
filtered off under nitrogen and washed with a little acetone and then with
diethyl ether. 1.05 g of an orange-coloured solid were obtained.
Hydrogenation with catalyst I
The catalyst (10 mg) was dissolved in toluene (25 ml) and added under argon
to the solution of a mixture (1.1 g) of avermectin B1a (96%) and avermectin
B1b (4%) and of 100 mg of triphenylphosphine in toluene (25 ml) in a
stainless steel autoclave. This starting material was then hydrogenated at
88°C under a hydrogen pressure of 20 bar with stirring of the solution. After
10 hours, HPLC analysis revealed a content of 86% dihydro-avermectin B1a
and of 4 % dihydroavermectin B1b, and also of 3% tetrahydroavermectin B1a.
Preparation of Catalyst II
Under an atmosphere of argon, a mixture of 7.5 mg of rhodium trichloride,
30.0 mg of tris-(hexylphenyl)-phosphine, 3 ml of acetone and 15 ml of
hydrazine hydrate is heated with stirring and reflux cooling for 4 hours.
Hydrogenation with catalyst II
The catalyst is added to a solution of 4.3 g of avermectin (B1a and B1b
mixture) in 25 ml of a mixture of acetone and cyclohexane in a ratio of 2:1.
After addition of 51.4 mg of tris-(mexylphenyl)phosphine, the hydrogenation
is carried out in a steel autoclave at a hydrogen pressure 5 bar and at 88°C.
After a hydrogenation time of 4 hours, 8.9% of starting material, 89.9% of
ivermectin (B1a and B1b mixture), tetrahydroavermectin content <0.1% was
obtained (according to HPLC analysis).
Removing of the catalyst system
The crude product after distillative removal of the solvent mixture, dissolved
in a mixture of 35 ml of methanol and 20 ml of water and this solution is
extracted with 25 ml of cyclohexane in a separating funnel. The phases are
separated and concentrated under reduced pressure. The extraction is
repeated twice in the same manner.
brand name
Stromectol (Merck);Mectizan.
Therapeutic Function
Antiprotozoal
Antimicrobial activity
It is also active against O. volvulus and other filarial worms, but the effect is chiefly directed against the larval forms (microfilariae). Uniquely among anthelmintic agents it exhibits activity against some ectoparasites, including Sarcoptes scabiei.
Biological Activity
Positive allosteric modulator of the α 7 neuronal nicotinic acetylcholine receptor and the purinergic P2X 4 receptor. Antihelmintic. Also modulates glutamate- and GABA-activated chloride channels. Potentiates glycine-gated currents at low concentrations (30 nM).
Mechanism of action
Two mechanisms of action are thought to be involved in the action of IVM. The first is an indirect action in which motility of microfalaria is reduced, which in turn allows cytotoxic cells of the host to adhere to the parasite, resulting in elimination from the host. This action may occur by virtue of the ability of IVM to act either as a γ-aminobutyric acid (GABA) agonist or as an inducer of chloride ion influx, leading to hyperpolarization and muscle paralysis. The chloride ion influx appears to be the more plausible mechanism. Recently, it has been shown that IVM binds irreversibly to the glutamate-gated chloride channel of the nematode Haemonchus contortus, whereas the channel is in an open conformation. The binding then remains locked in the open conformation, allowing ions to cross the membrane, leading to the paralytic action of IVM. The result of this action is a rapid decrease in microfilarial concentrations.
A second action of IVM leads to the degeneration of microfilariae in utero. This action would result in fewer microfilariae being released from the female worms, and it occurs over a longer period of time. The presence of degenerated microfilariae in utero prevents further fertilization and production of microfilariae.
Pharmacokinetics
Oral absorption: c. 60%
Cmax 12 mg oral: c. 30–47 ng/mL after 4 h
Plasma half-life: c. 12 h
Volume of distribution: 46.9 L
Plasma protein binding: 93%
It is rapidly metabolized in the liver and the metabolites are excreted in the feces over about 12 days with minimal (<1%) urinary excretion. Highest concentrations occur in the liver and fat. Extremely small amounts are found in the brain.
Clinical Use
Ivermectin has broad-spectrum activity in that it can affect nematodes, insects, and acarine parasites. It is the drug of choice in onchocerciasis and is quite useful in the treatment of other forms of filariasis, strongyloidiasis, ascariasis, loiasis, and cutaneous larva migrans. It is also highly active against various mites. It is the drug of choice in treating humans infected with Onchocerca volvulus, acting as a microfilaricidal drug against the skin-dwelling larvae (microfilaria). Annual treatment can prevent blindness from ocular onchocerciasis. Ivermectin is clearly more effective than diethylcarbamazine in bancroftian filariasis, and it reduces microfilaremia to near zero levels. In brugian filariasis diethylcarbamazine- induced clearance may be superior. It also is used to treat cutaneous larva migrans and disseminated strongyloidiasis. Its safe use in pregnancy has not been fully established.
Clinical Use
Ivermectin (Cardomec, Eqvalan, Ivomec) is a mixtureof 22,23-dihydro derivatives of avermectins B1a and B1bprepared by catalytic hydrogenation. Avermectins aremembers of a family of structurally complex antibioticsproduced by fermentation with a strain of Streptomycesavermitilis. Their discovery resulted from an intensivescreening of cultures for anthelmintic agents from naturalsources. Ivermectin is active in low dosage against awide variety of nematodes and arthropods that parasitizeanimals.
Ivermectin has achieved widespread use in veterinarypractice in the United States and many countries throughoutthe world for the control of endoparasites and ectoparasitesin domestic animals. It has been found effective forthe treatment of onchocerciasis (“river blindness”) in humans, an important disease caused by the roundwormOncocerca volvulus, prevalent in West and Central Africa,the Middle East, and South and Central America.Ivermectin destroys the microfilariae, immature forms ofthe nematode, which create the skin and tissue nodules thatare characteristic of the infestation and can lead to blindness.It also inhibits the release of microfilariae by theadult worms living in the host. Studies on the mechanismof action of ivermectin indicate that it blocks interneuron–motor neuron transmission in nematodes by stimulatingthe release of the inhibitory neurotransmitter GABA.The drug has been made available by the manufacturer ona humanitarian basis to qualified treatment programsthrough the World Health Organization.
Clinical Use
Onchocerciasis
Non-disseminated strongyloidiasis
Lymphatic filariasis (in combination with albendazole)
Scabies
If the patient is harboring Asc. lumbricoides, the worms will be passed in the feces. Head lice will also be killed, which is very much welcomed by the treated patients. Ivermectin has been widely used in the veterinary field, where use is also made of its effect on ectoparasites.
Side effects
In the treatment of onchocerciasis mild Mazzotti-type reactions occur, with occasional neurological problems. Although it is highly effective against L. loa, care must be taken to avoid treating patients with high microfilarial counts: there is one report of a patient with a concomitant L. loa infection who died when treated for onchocerciasis. Mild gastrointestinal and nervous system signs may occur following treatment for strongyloidiasis.
Side effects
The side effects are minimal, with pruritus, fever, and tender lymph nodes occasionally seen. The side effects are considerably less than those associated with diethylcarbamazine administration.
Veterinary Drugs and Treatments
Ivermectin is approved in horses for the control of: large strongyles
(adult) (Strongylus vulgaris, S. edentatus, S. equinus, Triodontophorus
spp.), small strongyles, pinworms (adults and 4th stage larva), ascarids
(adults), hairworms (adults), large-mouth stomach worms
(adults), neck threadworms (microfilaria), bots (oral and gastric
stages), lungworms (adults and 4th stage larva), intestinal threadworms
(adults), and summer sores (cutaneous 3rd stage larva) secondary
to Hebronema or Draschia Spp.
In cattle, ivermectin is approved for use in the control of gastrointestinal
roundworms (adults and 4th stage larva), lungworms
(adults and 4th stage larva), cattle grubs (parasitic stages), sucking
lice, and mites (scabies). For a listing of individual species covered,
refer to the product information.
In swine, ivermectin is approved for use to treat GI roundworms,
lungworms, lice, and mange mites. For a listing of individual species
covered, refer to the product information.
In reindeer, ivermectin is approved for use in the control of
warbles.
In American Bison, ivermectin is approved for use in the control
of grubs.
In dogs and cats, ivermectin is approved only for use as a preventative
for heartworm. It has also been used as a microfilaricide,
slow-kill adulticide, ectoparasiticide, and endoparasiticide.
Metabolism
Ivermectin is rapidly absorbed, is bound to a great extent to plasma protein, and is excreted in the urine or feces either unchanged or as the 3′-O-demethyl-22,23-dihydroavermectin B1α or as the dihydroavermectin B1α monosaccharide. The absorption of IVM is significantly affected by the presence of alcohol. Administration of IVM as an alcoholic solution may result in as much as a 100% increase in absorption.
storage
+4°C
Toxicity evaluation
Ivermectin toxicity has been reported in collie dogs and may be due to increased penetration of drug across the blood-brain barrier to the central nervous system (91) and/or the release of γ -aminobutyric acid in the central nervous system (92). Vomiting, salivation, diarrhea, melena, and death have resulted when dogs with Dirofilaria immitis microfilariae were treated with ivermectin (93,94). Adverse reactions in horses with Onchocerca cervicalis microfilariae at the time of therapy may manifest as transient, ventral, subcutaneous edema (95).
References
References/Citations 1) Wagstaff?et al.?(2012),?Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus; Biochem. J.,?443?851 2) Caly?et al.?(2020),?The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro; Antiviral Res.,?178?104787 3) Ottesen and Campbell (1994),?Ivermectin in human medicine; J. Antimicrob. Chemother.,?34?195
Ivermectin Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei ZB Gamay Biological Technology Co.,Ltd | +8617330018573 | info@zbvet.net | China | 245 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8816 | 58 |
| Hebei Miaoyin Technology Co.,Ltd | +86-17367732028 +86-17367732028 | kathy@hbyinsheng.com | China | 3512 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12829 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 | Sibel@weibangbio.com | China | 6087 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5986 | 58 |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | Jerry@xhobio.com | China | 484 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
Related articles
- Ivermectin: Indications, Onset of action, Mechanism of action and Side Effects
- Ivermectin is an antiparasitic drug approved by the FDA for the treatment of several tropical diseases, including onchocercias....
- Jun 4,2024
- What are the functions of Ivermectin?
- Ivermectin is a remarkably potent anthelmintic and insecticide
- Feb 18,2024
- Ivermectin–Old Drug,New Tricks?
- Ivermectin is an antiinfective agent with activity against several parasitic nematodes and scabies and is the treatment of cho....
- Feb 16,2022
Related Qustion
- Q:Can Ivermectin be used to treat COVID-19?
- A:No. First, ivermectin is not approved or authorized by the FDA for the treatment of COVID-19, and ivermectin is not approved b....
- Dec 18,2023
View Lastest Price from Ivermectin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-31 | Ivermectin
70288-86-7
|
US $50.00 / kg | 1kg | 99.9% | 10000 Kilogram/Kilograms per Day | Hebei Miaoyin Technology Co.,Ltd | |
 |
2024-10-31 | Ivermectin
70288-86-7
|
US $100.00-75.00 / kg | 1kg | 99% | 5000Ton | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-10-31 | Ivermectin
70288-86-7
|
US $10.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- Ivermectin
70288-86-7
- US $50.00 / kg
- 99.9%
- Hebei Miaoyin Technology Co.,Ltd
-

- Ivermectin
70288-86-7
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Ivermectin
70288-86-7
- US $10.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd