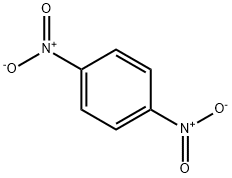
1,4-DINITROBENZENE
- CAS No.
- 100-25-4
- Chemical Name:
- 1,4-DINITROBENZENE
- Synonyms
- P-DINITROBENZENE;p-dinitro-benzen;1,4-Dinitrobenzol;nsc3809;1.4-Dinitr;dithanea-4;Dithane a-4;p-dinitrlbinzene;4-Dinitrobenzene;1,4-dinitrlbenzene
- CBNumber:
- CB3152579
- Molecular Formula:
- C6H4N2O4
- Molecular Weight:
- 168.11
- MDL Number:
- MFCD00007314
- MOL File:
- 100-25-4.mol
- MSDS File:
- SDS
| Melting point | 170-173 °C(lit.) |
|---|---|
| Boiling point | 183.4 °C34 mm Hg(lit.) |
| Density | 1.625 g/mL at 25 °C(lit.) |
| vapor pressure | 2.25 x 10-4 mmHg at 35 °C (Hine et al., 1963) |
| refractive index | 1.725 (589.3 nm) |
| Flash point | 150 °C |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble1g in 300ml |
| form | Crystals or Powder |
| color | Ochre to orange |
| Water Solubility | Soluble in water. (0.8 g/L) at 20°C. |
| Merck | 14,3273 |
| BRN | 1105828 |
| Henry's Law Constant | 4.79(x 10-7 atm?m3/mol) at 35 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: TWA 1, IDLH 50; OSHA PEL: TWA 1 ACGIH TLV: TWA 0.15 ppm for all isomers (adopted). |
| Stability | Stable, but may be shock-sensitive. May explode if heated. Incompatible with oxidizing agents, strong bases, nitric acid, many metals, tin oxides. |
| CAS DataBase Reference | 100-25-4(CAS DataBase Reference) |
| EWG's Food Scores | 1-2 |
| FDA UNII | 784Q9O56S9 |
| Proposition 65 List | p-Dinitrobenzene |
| EPA Substance Registry System | p-Dinitrobenzene (100-25-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H373-H410 | |||||||||
| Precautionary statements | P262-P264-P273-P280-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N,T,F | |||||||||
| Risk Statements | 26/27/28-33-34-50/53-52/53-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 28-36/37-45-60-61-16 | |||||||||
| RIDADR | UN 3443 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CZ7525000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29042090 | |||||||||
| Toxicity | IC50 (24-h) for river bacteria 1.27 mg/L (Yuan and Lang, 1997). | |||||||||
| NFPA 704 |
|
1,4-DINITROBENZENE Chemical Properties,Uses,Production
Chemical Properties
light yellow powder
Physical properties
Clear, colorless to white crystalline solid or monoclinic crystals. Slowly turns yellow on exposure to air.
Uses
1,4-Dinitrobenzene is used in a study to evaluate the ionization mechanism and solvent effect by novel atmospheric pressure photoionization mass spectrometry in negative ion mode for analysis of some compounds. 1,4-Dinitrobenzene can be used in synthesis of dyes and dye intermediates.
Uses
manufacture of dyes, dye intermediates, explosives, plastics.
Definition
ChEBI: A dinitrobenzene carrying nitro groups at positions 1 and 4.
General Description
Colorless to yellow solid. Sinks and mixes slowly with water.
Air & Water Reactions
Slowly mixes with water.
Reactivity Profile
All three isomers have similar properties and may react vigorously with oxidizing materials. Their reaction with nitric acid (nitration) will lead to a mixture of trinitrobenzenes possessing high-explosive properties [Urbanski, 1967, vol. 3, p. 290]. If heat and reaction conditions of the nitration are not controlled, detonation comparable to TNT may occur [Anon., J. R. Inst. Chem., 1960, 84, p. 451]. Mixture of 1,3-dinitrobenzene with tetranitromethane was found highly explosive [Urbanski, 1964, vol. 1, 592]. 1,2-dinitrobenzene is a severe explosion hazard when shocked or exposed to heat or flame. When heated to decomposition all dinitrobenzens emit toxic fumes of nitrogen oxides [Sax, 9th ed., 1996, p. 1374].
Health Hazard
INHALATION OR INGESTION: Headache, vertigo, nausea, vomiting, diarrhea, fever, rapid weak pulse, decreased blood pressure, cyanosis, exhaustion, hepatomegaly, jaundice, albuminurea, hematuria, visual scotomata, amblyopia and nystagmus. EYES: Irritation. SKIN: Stains skin yellow; if skin contact is prolonged, can be absorbed into blood causing same symptoms as for inhalation.
Safety Profile
Suspected carcinogen. Poison by ingestion. Mutation data reported. Mxture with nitric acid is a high explosive. When heated to decomposition it emits toxic fumes of NOx. See also 0and mDINITROBENZENE
Environmental Fate
Biological. In activated sludge inoculum, following a 20-d adaptation period, no biodegradation
was observed (Pitter, 1976).
Photolytic. Low et al. (1991) reported that the nitro-containing compounds (e.g., 2,4-
dinitrophenol) undergo degradation by UV light in the presence of titanium dioxide yielding
ammonium, carbonate, and nitrate ions. By analogy, 1,4-dinitrobenzene should degrade forming
identical ions.
Chemical/Physical. Releases toxic nitrogen oxides when heated to decomposition (Sax and
Lewis, 1987). 1,4-Dinitrobenzene will not hydrolyze in water (Kollig, 1993).
Purification Methods
Crystallise 1,4-dinitrobenzene from EtOH or EtOAc. Dry it under vacuum over P2O5. It can be sublimed in a vacuum. [Beilstein 5 IV 741.]
1,4-DINITROBENZENE Preparation Products And Raw materials
100-25-4(1,4-DINITROBENZENE)Related Search:
1of4