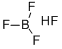Fluoroboric acid
- CAS No.
- 16872-11-0
- Chemical Name:
- Fluoroboric acid
- Synonyms
- TETRAFLUOROBORIC ACID;FLUOBORIC ACID;HYDROFLUOROBORIC ACID;HYDROGEN TETRAFLUOROBORATE;tetrafluoroboric acid solution;48 en tetrafL;fluoroboric;borofluorid;Borofiuoric acid
- CBNumber:
- CB6853202
- Molecular Formula:
- BF3.FH
- Molecular Weight:
- 87.81
- MDL Number:
- MFCD00011345
- MOL File:
- 16872-11-0.mol
- MSDS File:
- SDS
| Melting point | -90°C |
|---|---|
| Boiling point | 130°C (dec.) |
| Density | 1.38 g/mL at 20 °C(lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 5 mmHg ( 20 °C) |
| refractive index | nD20 of a 20% aq soln 1.3284 |
| Flash point | −40 °F |
| solubility | very soluble in H2O, ethanol |
| pka | -4.9[at 20 ℃] |
| form | Liquid |
| color | Light Yellow |
| Odor | Odorless |
| Water Solubility | MISCIBLE |
| Merck | 14,4149 |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| Stability | Stable. Incompatible with strong bases, cyanides. |
| CAS DataBase Reference | 16872-11-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | H429WZ9FBQ |
| EPA Substance Registry System | Fluoroboric acid (16872-11-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P280-P301+P330+P331-P303+P361+P353-P305+P351+P338+P310 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45-37-27 | |||||||||
| RIDADR | UN 1775 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | ED2685000 | |||||||||
| F | 3 | |||||||||
| Hazard Note | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28111990 | |||||||||
| NFPA 704 |
|
Fluoroboric acid price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 207934 | Tetrafluoroboric acid solution 48 wt. % in H2O | 16872-11-0 | 25g | $69.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 207934 | Tetrafluoroboric acid solution 48 wt. % in H2O | 16872-11-0 | 500g | $140 | 2024-03-01 | Buy |
| Alfa Aesar | 011484 | Tetrafluoroboric acid, 48% min w/w aq. soln. | 16872-11-0 | 50g | $50.4 | 2023-06-20 | Buy |
| Alfa Aesar | 011484 | Tetrafluoroboric acid, 48% min w/w aq. soln. | 16872-11-0 | *4x500g | $192 | 2023-06-20 | Buy |
| Strem Chemicals | 93-0503 | Tetrafluoroboric acid, 48% aqueous solution | 16872-11-0 | 500g | $81 | 2024-03-01 | Buy |
Fluoroboric acid Chemical Properties,Uses,Production
Outline
Fluoroboric acid is also known as "hydrogen fluoroboric acid," "tetrafluoroborate boron-hydrogen acid." Chemical formula is HBF4. Molecular weight is 87.81. It is transparent colorless liquid, toxic, has a strong corrosion, it can not be stored in a glass container for a long time. Boiling point is 130 ℃, while it decomposes slowly. Dissolved in water and ethanol, thermal decomposition with water to generate oxygen fluorine boric acid and boron trioxide (may be concentrated to 30%), the solution was strongly acidic, it does not corrode glass at room temperature. Fluoroboric acid is a strong acid, only exists in solution, the common fluoroborate is potassium tetrafluoroborate.
Method: Can be prepared by the reaction of Concentrated hydrofluoric acid and boric acid.
Uses: used in light metal smelting and electroplating, etc. Even very dilute solution can also be used as fermentation inhibitors, also used for preparing diazonium salts, acetal catalyst, used as an agent for determination of sodium in the presence of magnesium and potassium ions. Aniline as raw materials in concentrated hydrochloric acid reacts with sodium nitrite to generate diazonium salt, then at a low temperature (-10 ℃), diazonium salt reacts with fluoroboric acid, final pyrolysis, can be prepared fluorophenyl.
The above information is edited by the chemicalbook of Yan Yanyong.
Toxicity
It has a strong corrosive effect on the skin, mucous membrane, has a irritative effect on the eyes and respiratory system. Even diluted to be very thin, it can hinder fermentation. Maximum allowable concentration is 2.5 mg/m3. If accidentally splashed on the eyes or body, flush eyes with water, wash contaminated body parts thoroughly with soap and water. In severe cases, sent the patient to the hospital. The operator must wear masks, rubber gloves and wear overalls.
Chemical Properties
Transparent colorless liquid. miscible with water or alcohol.
Uses
Used as acetaldehyde synthesis catalysts, the metal surface cleaning agents, lead electrolytic polishing agents, the diazonium salt stabilizer, etc.
Used as Diazonium salts stabilizer, but also for electrolysis industry, etc.
Used for cleaning metal surface oxide, silicate film and as a corrosive, for cleaning before aluminum and alloy electroplating. 2.5% solution is for electrolytic polishing of pure aluminum and used as rewashing lotion for removing flux and electroplating parts from the metal substrate, as a catalyst for alkylation and polymerization, preservatives, chemical reagents, as well as the starting materials for preparing various fluoborates.
Used to clean metal and alloy surfaces prior to plating.
Used for preparing stabilized diazonium salts, manufacturing acetal catalyzed, used as an agent for determination of sodium in the presence of potassium ions and magnesium ions, a catalyst in organic synthesis, but also used for the sponge titanium and its alloys-dissolution and the printed wiring board industry.
Production method
Hydrofluoric acid method is according to the theoretical amount of hydrofluoric acid and boric acid ingredients, slowly adding boric acid to hydrofluoric acid under stirring, the reaction temperature was controlled below 40 ℃, stop mechanical stirring upon heating completed, placed at room temperature for more than 2h, then filtered and purified to obtain fluoroborate finished production.
4HF + H3BO3 → HBF3OH + HF + 2H2O
HBF3OH + HF → HBF4 + H2O
Category
Corrosive materials
Toxicity grading
Poisoning
Flammability hazard characteristics
In case of hair H combustible pore-forming agent; thermal decomposition of toxic fluoride gas.
Storage Characteristics
Treasury ventilation low-temperature drying, stored separately from Cyanide, 3,7-dinitroso-1,3,5,7-tetraazobicyclo-nonane, alkalis.
Extinguishing agent
Sand, carbon dioxide
Professional standards
TWA 2.5 mg (fluorine)/cubic meter
Description
Fluoboric acid is a colorless liquid which doesnot exist as a free, pure substance. Used as an aqueous solution. Molecular weight=87.82; Boiling point=130C(decomposes). Hazard Identification (based on NFPA-704M Rating System): Health 3, Flammability 0, Reactivity 0.Soluble in water.
Chemical Properties
Fluoboric acid is a colourless liquid, completely solvable in water. Fluoboric acid is a stable chemical substance and extremely reactive or incompatible with strong bases, cyanides. It is very corrosive in the presence of steel, aluminium, zinc, and copper and highly corrosive in the presence of glass and stainless steel.
Chemical Properties
Fluoboric acid is a colorless liquid which does not exist as a free, pure substance. Used as an aqueous solution.
Uses
Tetrafluoroboric acid is used in conjunction with various methods of cleaning and treat the surface of substrates. It is used as radiotracers.
Uses
As catalyst for preparing acetals, esterifying cellulose; to clean metal surfaces before welding; to brighten aluminum; as a solute in electrolytes for plating metals such as chromium, iron, nickel, copper, silver, zinc, cadmium, indium, tin, and lead (has a high throwing power). Reagent for sodium in the presence of magnesium and potassium ions; for making stabilized diazo salts (diazonium and tetrazonium fluoborates). An 0.1 to 0.5% solution retards fermentation: Homeyer, Pharm. Ztg. 34, 761 (1889).
Definition
ChEBI: Tetrafluoroboric acid is a boron fluoride. It is a conjugate acid of a tetrafluoroborate(1-).
General Description
A colorless odorless poisonous liquid. Boiling point 130°C. Corrosive to metals and tissue. Fluoroboric acid is used in electroplating, metal cleaning and making diazo salts.
Air & Water Reactions
Soluble in water with release of heat.
Reactivity Profile
Fluoroboric acid is a strong acid. Reacts exothermically with chemical bases (examples: amines, amides, and inorganic hydroxides). These reactions can generate dangerously large amounts of heat in small spaces. Dissolution in water or the dilution of a concentrated aqueous solution may generate significant heat. Reacts with active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. Generates flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. May catalyze (increase the rate of) chemical reactions. Attempted drying of the acid with acetic anhydride caused an explosion at 0°C [J. Organomet. Chem., 1975, 94, 319].
Hazard
Highly toxic, corrosive, irritant.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Safety Profile
A corrosive acid. When heated to decomposition it emits toxic vapors of B and F-.
Potential Exposure
Used as a catalyst for acetal synthesis and cellulose esters; a metal surface cleaning agent; an alu minum electrolytic finishing agent; a stripping solution for the removal of solder and plated metals; and an intermedi ate in making fluoroborate salt.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Prior toworking with this chemical you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from chemicallyactive metals and strong bases. Where possible, automatically pump liquid from drums or other storage containers toprocess containers.
Shipping
UN1775 Fluoroboric acid, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crystallise fluoroboric acid several times from conductivity water. It can be stored in a glass vessel at room temperature. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 221-222 1963.]
Incompatibilities
A strong acid. Reacts violently with chemically active metals; strong bases, releasing flammable hydrogen gas.
Fluoroboric acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Tangshan Moneide Trading Co., Ltd. | +86-315-8309571 +8615633399667 | sales@moneidechem.com | China | 574 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12465 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5873 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
Related articles
- How to synthesize Fluoroboric acid?
- Fluoroboric acid is an inorganic, corrosive, strong acid with the chemical formula [H+][BF4-]. Available in various quantities....
- May 21,2024
- Fluoroboric acid: properties, applications and safety
- Fluoroboric acid is a strong Lewis acid, forms stable complexes, used in dental implants and PZT etching. However, corrosive a....
- Jul 31,2023
- Fluoroboric acid-Uses in Organic Analysis
- Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula [H+....
- Jan 10,2020
View Lastest Price from Fluoroboric acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-08-29 | Fluoroboric acid
16872-11-0
|
US $13.00 / kg | 1kg | 50% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-08-15 | Fluoroboric acid
16872-11-0
|
US $30.00-5.00 / kg | 1kg | 0.99 | 10 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2024-01-08 | Fluoboric Acid
16872-11-0
|
US $6.00 / kg | 1kg | 40.0% , 48%-50% | 5000ton | Wuhan Boyuan Import & Export Co., LTD |
-

- Fluoroboric acid
16872-11-0
- US $13.00 / kg
- 50%
- Hebei Dangtong Import and export Co LTD
-

- Fluoroboric acid
16872-11-0
- US $30.00-5.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Fluoboric Acid
16872-11-0
- US $6.00 / kg
- 40.0% , 48%-50%
- Wuhan Boyuan Import & Export Co., LTD
16872-11-0(Fluoroboric acid)Related Search:
1of4