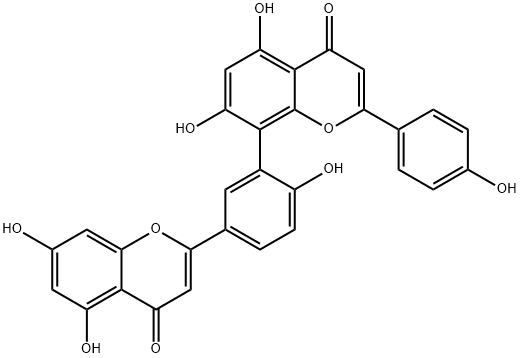
Amentoflavone
- CAS No.
- 1617-53-4
- Chemical Name:
- Amentoflavone
- Synonyms
- AMENTOFLAVONE;AMENTOFLACONE;amenthoflavone;I3,II8 BIAPIGENIN;3',8''-BIAPIGENIN;AMENTOFLAVONE(SH);Amentoflavone >AMENTOFLAVONE hplc;DIDEMETHYL-GINKGETIN;AMentotaxus biflavone
- CBNumber:
- CB3744024
- Molecular Formula:
- C30H18O10
- Molecular Weight:
- 538.46
- MDL Number:
- MFCD00017470
- MOL File:
- 1617-53-4.mol
| Melting point | >300°C (dec.) |
|---|---|
| Boiling point | 910.5±65.0 °C(Predicted) |
| Density | 1.656±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated), Pyridine (Slightly) |
| pka | 6.01±0.40(Predicted) |
| color | Yellow |
| BRN | 380244 |
| InChIKey | YUSWMAULDXZHPY-UHFFFAOYSA-N |
| LogP | 3.492 (est) |
| CAS DataBase Reference | 1617-53-4(CAS DataBase Reference) |
| FDA UNII | 9I1VC79L77 |
Amentoflavone price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 40584 | Amentoflavone ≥98.0% (HPLC) | 1617-53-4 | 1MG | $102 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHL80351 | Amentoflavone phyproof? Reference Substance | 1617-53-4 | 10MG | $288 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40584 | Amentoflavone ≥98.0% (HPLC) | 1617-53-4 | 5MG | $258 | 2024-03-01 | Buy |
| Sigma-Aldrich | 18571 | Amentoflavone analytical standard | 1617-53-4 | 10mg | $302.8 | 2024-03-01 | Buy |
| TCI Chemical | A2544 | Amentoflavone | 1617-53-4 | 20MG | $248 | 2021-12-16 | Buy |
Amentoflavone Chemical Properties,Uses,Production
Description
Amentoflavone , a bisapigenin , is one of the best inhibitors in the class of flavonoids , since its active dose is about 0.12uM.
Amentoflavone (C30H18O10) is a well-known biflavonoid occurring in many natural plants. This polyphenolic compound has been discovered to have some important bioactivities, including anti-inflammation, anti-oxidation, anti-diabetes, and anti-senescence effects on many important reactions in the cardiovascular and central nervous system, etc. Over 120 plants have been found to contain this bioactive component, such as Selaginellaceae, Cupressaceae, Euphorbiaceae, Podocarpaceae, and Calophyllaceae plant families.
Amentoflavone is a natural product with several associated biological effects.Its ability to block NF-xβ is the key for its anti-inflammatory potential. BETs were identified as NF-xβ promoters, with JQ-1 being highly effective in psoriasis models.
Biochem/physiol Actions
Biflavonoid with anti-inflammatory, anti-viral and cancer chemopreventive activity. It inhibits vascularization of tumors by blocking the activity of angiogenic VEGFs. Blocks the induction of COX-2 and up-regulates PPAR-γ. It is a negative modulator of the GABAA receptor at the benzodiazepine binding site.
Biological Functions
Amentoflavone is a biflavonoid originally isolated from Selaginella. It has a wide variety of biological effects including antibacterial, antioxidant, antiviral, antidiabetic, and neuroprotective activities. Amentoflavone has antiviral activity against the influenza A subtypes H1N1 and H3N2, influenza B, and herpes simplex virus 1 (EC50s = 3.1 and 4.3, 0.56, and 17.9 µg/ml, respectively). It has antidiabetic effects such that it dose-dependently increases insulin receptor phosphorylation and activation and inhibits hydrolysis of p-nitrophenyl phosphate (p-NPP) catalyzed by protein tyrosine phosphatase 1B (PTP1B; IC50 = 7.3 µM). Amentoflavone reduces the time mice spend immobile in the forced swim test, a measure of antidepressant efficacy, in a dose-dependent manner.
Synthesis of Amentoflavone
Amentoflavone, a biflavanoid, is ubiquitously found in plants such as Calophyllum inophyllum, Eucommia ulmoides, Selaginella doederleinii, Paulownia tomentosa var. tomentosa, Ginkgo biloba, Juglans sigillata, Hypericum perforatum. A wide variety of bioactivities such as anti-viral, anti-inflammatory, anti-tumor, antidepressant, anti-oxidant, anti-microbial, analgesic, antiplasmodial, leishmanicidal, lowering blood lipid and hepatoprotective activities have been reported for amentoflavone and its derivatives. Due to the limited natural abundance, the massive production of amentoflavone is not possible from natural resources. Therefore, total synthesis of amentoflavone would be significant as it will be able to solve the availability issue of amentoflavone. Although the synthesis of amentoflavone through Suzuki-reaction was reported two decades ago, which was to link the flavonyl-8-boronic acid with the 3'-iodoflavone to produce amentoflavone, no synthetic effort has been made ever since to explore an alternative scheme such that the flavonyl3'-boronic acid ester can be linked to the 8-iodoflavone through Suzuki coupling. It would be highly beneficial to the scientific community if this alternative scheme is successful, as this will provide a similar but different route for the synthesis of amentoflavone and other similar biflavonoids, because the preparation of flavonylboronic acid, the key intermediate for the synthesis of biflavonoids, from the corresponding halogenated flavone is sometimes problematic due to steric hindrance or unfavorable electronic effects from neighboring substituting groups in the aromatic ring. Therefore, the goal of this work is to provide an alternative synthetic scheme for the production of amentoflavone and other similar biflavonoids utilizing the coupling of flavonyl-3'-boronic acid ester and 8-iodoflavone, instead of the reported method which was based on the coupling of two different intermediates, the flavonyl-8- boronic acid and the 3'-iodoflavone [10]. Here we describe an efficient synthetic pathway to generate amentoflavone.
https://www.hilarispublisher.com/open-access/total-synthesis-of-amentoflavone-2161-0444-1000302.pdf
References
Amentoflavone (C30H18O10) is a common biflavonoid chemically named as 8-[5-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, which naturally occurs in many plants. It is also considered as an apigenin dimer linked by a C3′-C8′′ covalent bond. This compound was firstly isolated by Okigawa and his colleagues in 1971 from three plants of the Selaginella species (Selaginella tamariscina (Beauv.) Spring, Selaginella nipponica, and Selaginella pachystachys) .
https://www.mdpi.com/1420-3049/22/2/299/htm
Description
Amentoflavone is a biflavonoid originally isolated from Selaginella. It has a wide variety of biological effects including antibacterial, antioxidant, antiviral, antidiabetic, and neuroprotective activities. Amentoflavone has antiviral activity against the influenza A subtypes H1N1 and H3N2, influenza B, and herpes simplex virus 1 (EC50s = 3.1 and 4.3, 0.56, and 17.9 μg/ml, respectively). It has antidiabetic effects such that it dose-dependently increases insulin receptor phosphorylation and activation and inhibits hydrolysis of p-nitrophenyl phosphate (p-NPP) catalyzed by protein tyrosine phosphatase 1B (PTP1B; IC50 = 7.3 μM). Amentoflavone reduces the time mice spend immobile in the forced swim test, a measure of antidepressant efficacy, in a dose-dependent manner.
Uses
Amentoflavone, is isolated from an Et acetate ext. of the whole plant of Selaginella tamariscina. It has been shown to have antitumor activity, such as mitochondria-mediated apoptotic cell death. Amentoflavone can interact with many medications by being a potent inhibitor of CYP3A4 and CYP2C9, which are enzymes responsible for the metabolism of some drugs in the body.
Definition
ChEBI: Amentoflavone is a biflavonoid that is obtained by oxidative coupling of two molecules of apigenin resulting in a bond between positions C-3 of the hydroxyphenyl ring and C-8 of the chromene ring. A natural product found particularly in Ginkgo biloba and Hypericum perforatum. It has a role as a cathepsin B inhibitor, an antiviral agent, an angiogenesis inhibitor, a P450 inhibitor and a plant metabolite. It is a biflavonoid, a hydroxyflavone and a ring assembly.
Biochem/physiol Actions
Biflavonoid with anti-inflammatory, anti-viral and cancer chemopreventive activity. It inhibits vascularization of tumors by blocking the activity of angiogenic VEGFs. Blocks the induction of COX-2 and up-regulates PPAR-γ. It is a negative modulator of the GABAA receptor at the benzodiazepine binding site.
Amentoflavone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 290 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| Chengdu Greenpure Biopharma CO.,Ltd | 18283602253 | jancyzheng@gcgreenpure.com | China | 952 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3424 | 58 |
| Nanjing Dolon Biotechnology Co.,Ltd. | 18905173768 | sales@dolonchem.com | CHINA | 2972 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
View Lastest Price from Amentoflavone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Amentoflavone
1617-53-4
|
US $0.00 / kg | 1kg | 10% - 90% | 3000 kg | BINBO BIOLOGICAL CO.,LTD | |
 |
2023-11-01 | Amentoflavone
1617-53-4
|
US $0.00-0.00 / mg | 1mg | HPLC>=98% | 10kg | Hangzhou ICH Biofarm Co., Ltd | |
 |
2023-07-28 | Amentoflavone
1617-53-4
|
US $0.00-0.00 / kg | 1kg | 0.99 | 50000kg | Hebei Kingfiner Technology Development Co. , Ltd. |
-

- Amentoflavone
1617-53-4
- US $0.00 / kg
- 10% - 90%
- BINBO BIOLOGICAL CO.,LTD
-

- Amentoflavone
1617-53-4
- US $0.00-0.00 / mg
- HPLC>=98%
- Hangzhou ICH Biofarm Co., Ltd
-

- Amentoflavone
1617-53-4
- US $0.00-0.00 / kg
- 0.99
- Hebei Kingfiner Technology Development Co. , Ltd.