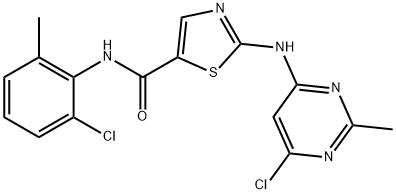
Dasatinib
- CAS No.
- 302962-49-8
- Chemical Name:
- Dasatinib
- Synonyms
- BMS-354825;CS-1968;Dasatinib;Dasaitinib;Dashatinib;NCGC00181129;25MG/100MG/1G;Dasatinib, 95+%;sprycel Dasatinib;Dasatinib Tablets
- CBNumber:
- CB4506696
- Molecular Formula:
- C22H26ClN7O2S
- Molecular Weight:
- 488.01
- MDL Number:
- MFCD11046566
- MOL File:
- 302962-49-8.mol
- MSDS File:
- SDS
| Melting point | 275-286°C |
|---|---|
| Density | 1.408±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 200 mg/ml). |
| pka | 10.94±0.70(Predicted) |
| form | White powder. |
| color | White or off-white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
| InChIKey | ZBNZXTGUTAYRHI-UHFFFAOYSA-N |
| SMILES | S1C(C(NC2=C(C)C=CC=C2Cl)=O)=CN=C1NC1C=C(N2CCN(CCO)CC2)N=C(C)N=1 |
| CAS DataBase Reference | 302962-49-8(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | BMS-354825; dasatinib |
| FDA UNII | X78UG0A0RN |
| NCI Drug Dictionary | dasatinib |
| ATC code | L01EA02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H318-H351-H361fd-H372-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| HS Code | 29341000 | |||||||||
| NFPA 704 |
|
Dasatinib price More Price(57)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML2589 | Dasatinib ≥98% (HPLC) | 302962-49-8 | 50MG | $48.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | CDS023389 | Dasatinib | 302962-49-8 | 25MG | $42.7 | 2024-03-01 | Buy |
| Cayman Chemical | 11498 | Dasatinib ≥98% | 302962-49-8 | 10mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 11498 | Dasatinib ≥98% | 302962-49-8 | 50mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 11498 | Dasatinib ≥98% | 302962-49-8 | 100mg | $78 | 2024-03-01 | Buy |
Dasatinib Chemical Properties,Uses,Production
Leukemia drug
Dasatinib is an oral potent oncogenic kinase inhibitor, which can block signal of cancer cell replication acceleration , in May 2009, the US Food and Drug Administration (FDA) formally approved the sale of dasatinib , It has been clinically used for the treatment of various chronic myeloid leukemia (CML), including treatment of chronic myeloid leukemia which is resistant or intolerant to the treating programs including imatinib mesylate., Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL) and the treatment of patients with solid tumors. 
October 28, 2010 ,the FDA approved dasatinib (Sprycel) of the new indication which is used for the treatment of the rare leukemia first diagnosed Philadelphia chromosome-positive called chronic myeloid leukemia (Ph + CP-CML) . The disease is a blood and bone marrow disease associated with genetic abnormality .
An open-label randomized clinical trial conducted in patients with CP-CML evaluated the safety and efficacy of dasatinib . Common side effects include decreased activity of the bone marrow caused by red blood cells, white blood cells and platelets decreased (myelosuppression), fluid retention, diarrhea, headache, musculoskeletal pain, and rash.
October 11, 2011, the US Food and Drug Administration (FDA) said the leukemia drug dasatinib (Sprycel, Bristol-Myers Squibb Company) might increase pulmonary arterial hypertension (PAH) risk. Pulmonary hypertension is a rare but serious disease that can lead to the condition that heart pumping blood to the lungs becomes more difficult by raising the pressure . PAH symptoms include shortness of breath, fatigue, swelling of the legs and ankles, clinicians must differentiate these symptoms from other similar symptoms.
Since 2006 Dasatinib was approved in the US market ,there had been 12 associated pulmonary hypertension events ,pulmonary hypertension symptoms included shortness of breath, fatigue, hypoxia and fluid retention, patients then were confirmed pulmonary hypertension by the results of right heart catheterization ,studies have shown that the biggest cause of the disease may come from patients taking dasatinib.
The above information is edited by the Chemicalbook of Tian Ye.
Chemical properties
Dasatinib is a white to off-white powder and has a melting point of 280°-286° C. The drug substance is insoluble in water and slightly soluble in ethanol and methanol. SPRYCEL™ (dasatinib) is an inhibitor of multiple tyrosine kinases.SPRYCEL tablets are white to offwhite, biconvex, film-coated tablets containing dasatinib, with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021986lbl.pdf
Mechanism of Action
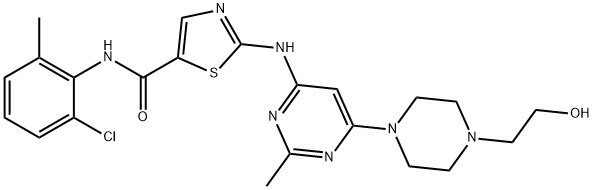
Dasatinib (former BMS 354825), or N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2- hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carbox- amide monohydrate (C22H26ClN7O2S), is an orally available small-molecule multitargeted kinase inhibitor. Dasatinib was discovered by and named after Jagabandhu Das (Lombardo et al. 2004; Das et al. 2006) as part of an effort to develop potent inhibitors of SRC family kinases (SFKs).
The compound targets the SRC family of kinases (SRC, LCK, HCK, YES, FYN, FGR, BLK, LYN, FRK). In addition, and clinically more significant, da- satinib inhibits BCR-ABL with greater potency compared to other BCR-ABL inhibitors.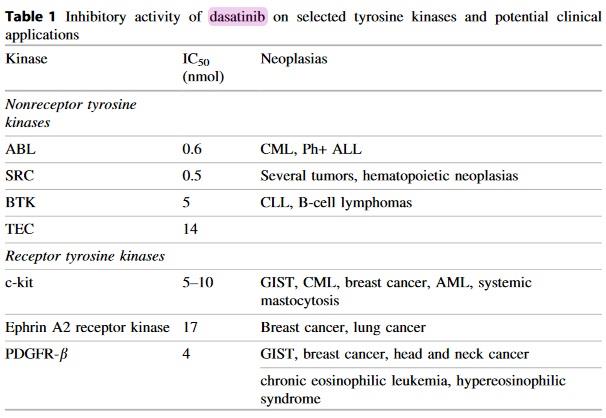
It also inhibits receptor tyrosine kinases (c-KIT, PDGFR, DDR1 and 2, c-FMS, ephrin receptors) and TEC family kinases (TEC and BTK) (Table 1).
Preclinical studies suggest that dasatinib induces apoptosis in only a small subset of cell lines. Inhibition of migration, invasion, and cell adhesion by da- satinib is reported more frequently (Johnson et al. 2005; Nam et al. 2005; Serrels et al. 2006). It has been demonstrated that dasatinib induces defects in spindle generation, cell cycle arrest, and centrosome alterations in leukemic cells, tumor cell lines, and also in normal cells. These effects are not attributable to the inhi- bition of a single kinase; rather it is expression of nonspecific effects on multiple kinases (Fabarius et al. 2008).
In a nude mouse model of prostate cancer, tumor growth and the development of lymph node metastasis were inhibited by dasatinib (Park et al. 2008). In addition, Dasatinib acts also on the tumoral microenvironment, especially in bone, where dasatinib inhibits osteoclastic activity and favors osteogenesis, exerting a bone-protecting effect (Metcalf et al. 2002).
Pharmacokinetics
Dasatinib is administered orally. The drug is rapidly absorbed, peak plasma concentrations occur 0.5-3 h after administration. The intake of food is not relevant for pharmacokinetics of dasatinib. In a dose range of 25-120 mg twice daily, the area under the plasma concentration—time curve (AUC) increased proportionally. The drug is extensively metabolized in the liver, predominantly by cytochrome P 450 (CYP) 3A4, only 30 % remain unchanged. The metabolites of the compound are unlikely to play a pharmacologic role. There were linear elimination characteristics over the above-mentioned dose range with a terminal elimination half-life of 5-6 h.
Elimination occurs mostly in the feces (85 %) only little in urine (4 %). Dasatinib is excreted as metabolites, only 19% of a dose was recovered as unchanged drug in the feces (SprycelÒBMS 2012).
Uses
Dasatinib is a novel, potent, and multi-targeted inhibitor that targets Abl, Src and c-Kit, with IC50 of <1 nM, 0.8 nM and 79 nM, respectively. It could directly targets wild-type and mutant c-Abl kinase domains. As a small-molecule Tyrosine Kinase Inhibitor (TKI) for the treatment of CML, dasatinib is used to treat a certain type of chronic myeloid leukemia (CML; a type of cancer of the white blood cells) as a first treatment and in people who can no longer benefit from other leukemia medications including imatinib (Gleevec) or in those who cannot take these medications because of side effects. It is also used to treat a certain type of chronic CML in children. Dasatinib could used to treat a certain type of acute lymphoblastic leukemia (ALL; a type of cancer of the white blood cells) in people who can no longer benefit from other leukemia medications or who cannot take these medications because of side effects. Dasatinib is in a class of medications called kinase inhibitors. It works by blocking the action of an abnormal protein that signals cancer cells to multiply. This helps stop the spread of cancer cells.
https://medlineplus.gov/druginfo/meds/a607063.html
Toxicology
Dasatinib has a unique safety profile and since early clinical trials some AEs have been consistently reported in patients receiving dasatinib including myelosup- pression, fluid retention, pleural effusion, gastrointestinal disorders, fatigue, headache, musculoskeletal disorders, rash, and infection (Table 8). Some bleeding events have also been reported. More recently, cases of pulmonary arterial hypertension (PAH), a subcategory of pulmonary hypertension (PH), have been reported in a small number of patients receiving dasatinib (Galie et al. 2009; McLaughlin et al. 2009; Fang et al. 2012). In clinical trials of first-line and second- line dasatinib, most AEs occurred within 12—24 months of treatment and were managed with dose modifications (Kantarjian et al. 2012; Shah et al. 2012; SprycelÒBMS 2012).
Small Molecules in Oncology
Drug Interactions
Dasatinib is a substrate and an inhibitor of CYP3A4. Therefore, there is a potential for interaction with other concomitantly administered drugs that are metabolized primarily by or modulate the activity of CYP3A4.
Systemic exposure to dasatinib is increased if it is coadministered with drugs that are inhibitors of CYP 3A4 (e.g., clarithromycin, erythromycin, itraconazole, ketoconazole).
If coadministered with drugs that induce CYP 3A4 (e.g., dexamethasone, phenytoin, carbamazepine, rifampicin, phenobarbital or Hypericum perforatum, also known as St. John’s Wort), dasatinib AUC is reduced. It was reduced by 82% when coadministered with rifampicin.
Dasatinib AUC was reduced when coadministered with H2-blockers/protonpump inhibitors, or antacids. Concomitant administration of famotidin reduced dasatinib AUC by 61%, coadministration of aluminum hydroxide by 55%.
Dasatinib is an inhibitor of CYP3A4. Substrates of CYP3A4 with a narrow therapeutic index should be administered with caution in patients receiving dasatinib. Drugs that rank among that list are alfentanil, astemizole, terfenadine, cyclosporine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus, or ergot alkaloid (ergotamine, dihydroergotamine) (SprycelÒBMS 2012).
Description
Dasatinib, developed and marketed by Bristol Myers, is the first approved oral tyrosine kinase inhibitor which binds to multiple conformations of ABL kinase for the treatment of two leukemia indications: chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Dasatinib is a highly potent, ATPcompetitive ATPcompetitive kinase inhibitor which, at nanomolar concentrations, inhibits BCR-ABL, SRC family, c-KIT, EPHA2 and PDGFR-B.
Chemical Properties
Pale-Yellow Solid
Originator
Bristol–Myers Squibb (US)
Definition
ChEBI: Dasatinib (anhydrous) is an aminopyrimidine that is 2-methylpyrimidine which is substituted at position 4 by the primary amino group of 2-amino-1,3-thiazole-5-carboxylic acid and at position 6 by a 4-(2-hydroxyethyl)piperazin-1-yl group, and in which the carboxylic acid group has been formally condensed with 2-chloro-6-methylaniline to afford the corresponding amide. A multi-targeted kinase inhibitor, it is used, particularly as the monohydrate, for the treatment of chronic, accelerated, or myeloid or lymphoid blast phase chronic myeloid leukemia. Note that the name 'dasatinib' is used to refer to the monohydrate (USAN) as well as to anhydrous dasatinib (INN). It has a role as a tyrosine kinase inhibitor, an anticoronaviral agent and an antineoplastic agent. It is a secondary amino compound, a tertiary amino compound, an organochlorine compound, an aminopyrimidine, a member of 1,3-thiazoles, a monocarboxylic acid amide, a N-arylpiperazine and a N-(2-hydroxyethyl)piperazine. It is a conjugate base of a dasatinib(1+).
brand name
Sprycel (Bristol-Myers Squibb).
General Description
Dasatinib is available in 20-, 50-, and 70-mg tablets fororal administration in the treatment of CML and ALL thatare Ph1 positive. Although dasatinib is more potent thanimatinib, bioavailability is much lower with values rangingbetween 14% to 34%. The agent is extensively metabolizedwith as many as 29 metabolites seen as result of oxidationby primarily CYP3A4 and phase II conjugation.The agent may act as an inhibitor of CYP3A4 andCYP2C8. Metabolism does give an active metabolite, but this accounts for only 5% of the total and is not believed tobe important for the overall activity of the agent. Dasatinibis 95% protein bound with a terminal half-life of 3 to5 hours. The majority of the drug and metabolites are eliminatedin the feces. The most common side effects are skinrash, nausea, diarrhea, and fatigue. Serious side effects includemyelosuppression appearing as neutropenia andthrombocytopenia, bleeding of the brain and GI tract, andfluid retention.
Synthesis
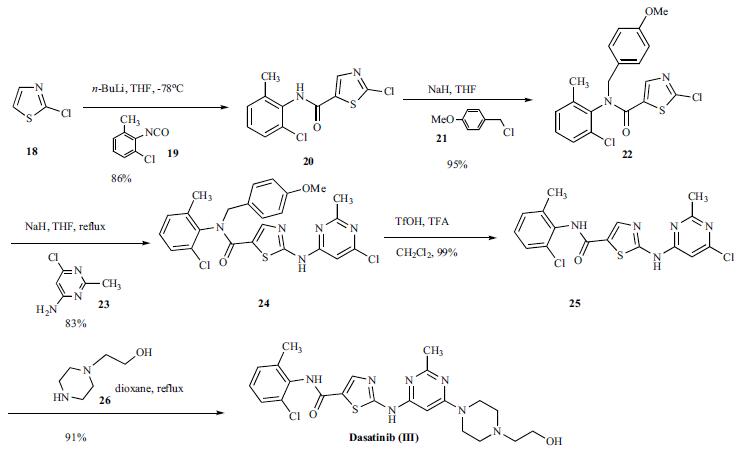
A concise and efficient route was developed for the synthesis of dasatinib. Reaction of 2-chlorothioa-zole (18) with n-butyllithium at low temperature followed by addition of 2-chloro-6-methylphenyl isocyanate (19) gave anilide 20 in 86% yield. The amide 20 was protected as corresponding 4-methoxy benzyl (PMB) anilide 22 in 95% yield which was subsequently reacted with 4- amino-6-chloro-2-methylpyrimidine (23) in the presence of sodium hydride in hot THF to give compound 24 in 83% yield. The PMB protecting group was then removed with triflic acid to give compound 25 in 99% yield. Compound 25 was reacted with 1-(2-hydroxyethyl)piperazine (26) in refluxing dioxane to give dasatinib (III) in 91% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antacids: absorption possibly reduced by antacids,
give at least 2 hours apart.
Antibacterials: metabolism accelerated by rifampicin
- avoid.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Antivirals: avoid with boceprevir.
Ulcer healing drugs: avoid with histamine H2
antagonists; concentration reduced by proton pump
inhibitors.
Metabolism
Dasatinib is extensively metabolised, mainly via the
cytochrome P450 isoenzyme CYP3A4, forming an active
metabolite.
Elimination is predominantly in the faeces, mostly as
metabolites. Following a single oral dose of [14C]-labelled
dasatinib, approximately 89% of the dose was eliminated
within 10 days, with 4% and 85% of the radioactivity
recovered in the urine and faeces, respectively. Unchanged
dasatinib accounted for 0.1% and 19% of the dose in
urine and faeces, respectively, with the remainder of the
dose as metabolites.
storage
Store at -20°C
References
1) Lombardo et al. (2004), Discovery of N-2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a duel Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays; J. Med. Chem., 47 6658 2) Nam et al. (2005), Action of the Src family kinase inhibitor dasatinib (BMS-354825), on human prostate cancer cells; Cancer Res., 65 9185 3) Johnson et al. (2005), Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells; Clin. Cancer Res., 11 6924
Dasatinib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Yangzhou Qinyuan Pharmatech Co.,ltd | +86-18752526868 | jennysun@yzqyyykj.com | China | 64 | 58 |
| Hefei TianRui Pharmaceutical Chemical Co., Ltd. | +86-0551-68665055 +86-+86-18616906106 | sales@trywchem.com | China | 129 | 58 |
| SHANDONG BOYUAN PHARMACEUTICAL CO., LTD. | +86-0531-69954981 +8615666777973 | dwyane.wang@boyuanpharm.com | China | 211 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 878 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
Related articles
- Dasatinib: Mechanism of action and Safety
- Dasatinib, a tyrosine kinase inhibitor, primarily treats chronic myeloid leukemia and Philadelphia chromosome-positive acute l....
- Mar 29,2024
- Dasatinib: BCR-ABL kinase inhibitor
- Dasatinib, known as Sprycel, is a receptor tyrosine kinase inhibitor developed by Bristol-Myers Squibb Company.
- Sep 20,2023
- Dasatinib: A second-generation BCR-ABL1 tyrosine kinase inhibitor
- Dasatinib is a tyrosine kinase inhibitor indicated for the treatment of chronic myeloid leukemia, but side effects may occur d....
- Sep 18,2023
View Lastest Price from Dasatinib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Dasatinib
302962-49-8
|
US $5.00 / kg | 1kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-22 | Dasatinib
302962-49-8
|
US $0.00 / KG | 2KG | USP26, EP VI / GMP DMF | 20tons | Sinoway Industrial co., ltd. | |
 |
2024-04-10 | Dasatinib
302962-49-8
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC |





