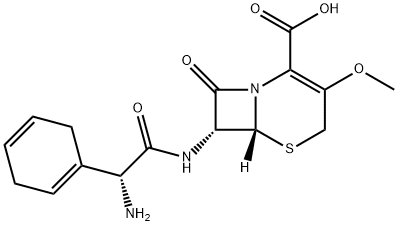
Cefroxadine
- CAS No.
- 51762-05-1
- Chemical Name:
- Cefroxadine
- Synonyms
- cxd;C12979;Oraspor;cgp9000;cefroxadin;CEFROXADINE;BRN 0587499;antibioticcgp9000;Cefroxadine. Oraspor;Cefroxadine USP/EP/BP
- CBNumber:
- CB5191790
- Molecular Formula:
- C16H19N3O5S
- Molecular Weight:
- 365.4
- MDL Number:
- MFCD01940013
- MOL File:
- 51762-05-1.mol
| Melting point | 170° (dec) |
|---|---|
| alpha | D20 +87° (c = 1.093 in 0.1N HCl) |
| Boiling point | 251°C (rough estimate) |
| Density | 1.3163 (rough estimate) |
| refractive index | 1.6390 (estimate) |
| pka | pKa 3.30±0.02(H2O t=35.0 I=0.00)(Approximate) |
| FDA UNII | B908C4MV2R |
| ATC code | J01DB11 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H334-H317 |
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Toxicity | LD50 in mice (mg/kg): >6000 orally; 7090 i.p. (Scartazzini, Bickel, 1978) |
Cefroxadine Chemical Properties,Uses,Production
Description
Cefroxadine was synthesized by Ciba-Geigy in 1972. A methoxyl group replaced the methyl group of cephradine at the 3 position of the cephem nucleus. Cefroxadine shows stronger activities than cephalexin, especially bactericidal and bacteriolytic activities, and it has better oral absorption that is less affected by a recent meal. Cefroxadine shows less renal toxicity than cephalexin in toxicological studies using animals.
Originator
Oraspor,Ciba Geigy,Switz.,1981
Uses
Antibacterial.
Definition
ChEBI: Cefroxadine is a first-generation cephalosporin antibiotic having methoxy and [(2R)-2-amino-2-(cyclohexa-1,4-dien-1-yl)acetyl]amino side groups located at positions 3 and 7 respectively.
Manufacturing Process
A suspension of 30.64 g (0.2 mol) of D-α-amino-α-(1,4-cyclohexadienyl)-
acetic acid in 600 ml of methylene chloride is cooled under a stream of argon
to 6°C, whereupon hydrogen chloride is passed in for about 30 minutes until
the mixture is saturated. Phosphorpentachloride (62.4 g, 0.3 mol) is added in
two portions. The mixture is stirred for 2 hours at 6°C to 8°C. The colorless
precipitate is filtered off under nitrogen and exclusion of moisture, washed
with methylene chloride and dried for 18 hours at 0.05 mm Hg at room
temperature to give D-α-amino-α-(1,4-cyclohexadienyl)-acetylchloride
hydrochloride in form of colorless crystals.
A suspension of 37.3 g (0.1 mol) of 7β-amino-3-methoxy-3-cephem-4-
carboxylic acid hydrochloride dioxanate in 500 ml methylene chloride is stirred
for 15 minutes at room temparature under an argon atmosphere and treated
with 57.2 ml (0.23 mol) of bis-(trimethylsilyl)acetamide. After 45 minutes the
faintly yellow slightly turbid solution is cooled to 0°C and treated within 10
minutes with 31.29 (0.15 mol) of D-α-amino-α-(1,4-cyclohexadienyl)-acetyl
chloride hydrochloride. Thirty minutes thereafter 15 ml (about 0.21 mol) of
propylene oxide is added and the mixture is further stirred for 1 hour at 0°C:
A cooled mixture of 20 ml of absolute methanol in 200 ml of methylene
chloride is added within 30 minutes, after another 30 minutes the precipitate
is filtered off under exclusion of moisture, washed with methylene chloride
and dried under reduced pressure at room temperature. The obtained
hygroscopic crystals of the hydrochloride of 7β-[D-α-(1,4-cyclohexadienyl)-
acetylamino]-3-methoxy-3-cephem-4-carboxylic acid are stirred into 200 ml of
ice water and the milky solution treated with about 66 ml of cold 2 N sodium
hydroxide solution until pH 3.5 is reached. The solution is clarified by filtration
through diatomaceous earth, washed with ice water, cooled to 0°C and treated
with 20 ml of 2 N sodium hydroxide solution until pH 5.7 is reached. A second
filtration through a glass filter frit results in a clear solution which is treated
with acetone (800 ml) at 0°C. The crystals are filtered washed with
acetone:water (2:1), acetone and diethyl ether and dried for 20 hours at
room temperature and 0.05 mm Hg to give the 7β-[D-α-amino-α-(1,4-
cyclohexadienyl)-acetylamino]-3-methoxy-3-cephem-4-carboxylic acid
dihydrate.
Therapeutic Function
Antibacterial
Antimicrobial activity
Cefroxadine is closely related to cefradine, the structure differing only by the presence of a methoxy group replacing methyl at the C-3 position. The antimicrobial spectrum is identical to that of cefradine and cefalexin. A dose of 1 g as film-coated tablets produced mean peak plasma levels of 25 mg/L at 1 h. Absorption is depressed and delayed by administration with food. The plasma elimination half-life is 0.8 h, rising to 40 h in end-stage renal failure and falling to 3.4 h during hemodialysis. Around 85% of an oral dose is excreted unchanged in the urine. It is available in Japan.
Cefroxadine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9126 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 28455 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 18958018566; | info@afinechem.com | China | 15377 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
View Lastest Price from Cefroxadine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-01 | Cefroxadine USP/EP/BP
51762-05-1
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited | |
 |
2020-05-26 | Cefroxadine
51762-05-1
|
US $0.00-0.00 / Kg | 1KG | 99.0% | 500 MT | Shaanxi Dideu Medichem Co. Ltd | |
 |
2019-07-06 | Cefroxadine
51762-05-1
|
US $7.00 / kg | 1kg | 99% | 100kg | Career Henan Chemical Co |
-

- Cefroxadine USP/EP/BP
51762-05-1
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Cefroxadine
51762-05-1
- US $0.00-0.00 / Kg
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
-

- Cefroxadine
51762-05-1
- US $7.00 / kg
- 99%
- Career Henan Chemical Co