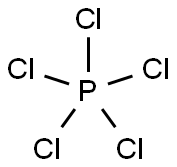
Phosphorus pentachloride
- CAS No.
- 10026-13-8
- Chemical Name:
- Phosphorus pentachloride
- Synonyms
- PCl5;PHOSPHORUS(V) CHLORIDE;Phosphorous pentachloride;PHOSPHORUS CHLORIDE;pentachlorophosphorane;phosphoricchloride;Fosforpentachloride;Phosphoric chloride;Phosphorus pentachL;pieciochlorekfosforu
- CBNumber:
- CB6423984
- Molecular Formula:
- Cl5P
Lewis structure
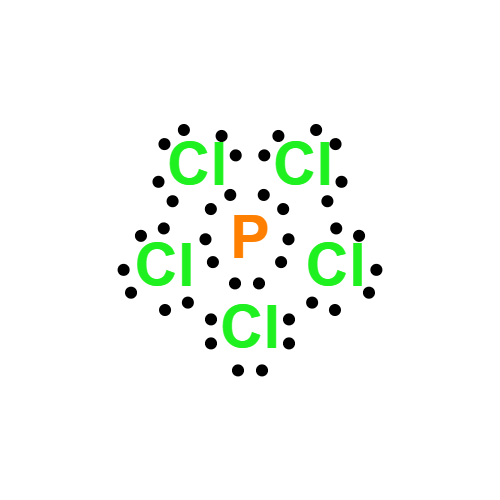
- Molecular Weight:
- 208.24
- MDL Number:
- MFCD00011439
- MOL File:
- 10026-13-8.mol
| Melting point | 179-181°C (subl.) |
|---|---|
| Boiling point | 160 °C |
| Density | 1.6 |
| vapor pressure | 0.016 hPa (20 °C) |
| storage temp. | 2-8°C |
| solubility | Soluble in carbon disulfide and carbon tetrachloride. |
| form | macroporous |
| color | Yellow |
| Specific Gravity | 1.6 |
| PH | 1 (5g/l, H2O)acidic |
| Odor | Pungent odour |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,7351 |
| Exposure limits | TLV-TWA 0.85 mg/m3 (0.1 ppm) (ACGIH), ~1 mg/m3 (0.1 ppm) (OSHA). . |
| Dielectric constant | 2.8(160℃) |
| Stability | Hygroscopic, Moisture Sensitive |
| InChIKey | UHZYTMXLRWXGPK-UHFFFAOYSA-N |
| CAS DataBase Reference | 10026-13-8(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 0EX753TYDU |
| NIST Chemistry Reference | Phosphorus pentachloride(10026-13-8) |
| EPA Substance Registry System | Phosphorus pentachloride (10026-13-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314-H330-H373 | |||||||||
| Precautionary statements | P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P314 | |||||||||
| Hazard Codes | T+ | |||||||||
| Risk Statements | 14-22-26-34-48/20 | |||||||||
| Safety Statements | 26-36/37/39-45-7/8 | |||||||||
| RIDADR | UN 1806 8/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 1 mg/m3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TB6125000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28121045 | |||||||||
| Toxicity | LD50 orally in Rabbit: 660 mg/kg | |||||||||
| IDLA | 70 mg/m3 | |||||||||
| NFPA 704 |
|
Phosphorus pentachloride price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22340 | Phosphorus pentachloride for synthesis | 10026-13-8 | 100g | $51.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22340 | Phosphorus pentachloride for synthesis | 10026-13-8 | 500g | $86.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22340 | Phosphorus pentachloride for synthesis | 10026-13-8 | 1kg | $145 | 2024-03-01 | Buy |
| Sigma-Aldrich | 157775 | Phosphorus pentachloride reagent grade, 95% | 10026-13-8 | 1kg | $118 | 2024-03-01 | Buy |
| Alfa Aesar | 010523 | Phosphorus(V) chloride, 98% | 10026-13-8 | 100g | $47.2 | 2023-06-20 | Buy |
Phosphorus pentachloride Chemical Properties,Uses,Production
Physical Properties
Yellowish-white tetragonal crystals; pungent odor; fumes in air; deliquescent; density 2.1 g/cm3; decomposes on heating; melts at 166.8°C under the pressure of its own vapor(triple point); sublimes at 160°C; critical temperature 373°C; hydrolyzes in water; soluble in carbon disulfide and carbon tetrachloride.
Uses
Phosphorus pentachloride is used as a chlorinating agent in many organic syntheses, such as production of alkyl and acid chlorides. It also is a catalyst in manufacturing acetylcellulose.
Preparation
Phosphorus pentachloride is prepared by reacting white phosphorus with excess dry chlorine. The white phosphorus is placed over sand in a retort from which air and moisture have been purged. The reaction is indicated by inflaming phosphorus:
P4 + 10Cl2 → 4PCl5
Also, the compound is obtained by reaction of dry chlorine with phosphorus trichloride:
PCl3 + Cl2 → PCl5
Reactions
Phosphorus pentachloride absorbs moisture from air forming phosphoryl chloride:
PCl5 + H2O → POCl3 + 2HCl
The above reaction is difficult to control and progresses to complete hydrolysis. Thus, in the presence of excess water or when treated with water, the pentachloride is hydrolyzed to phosphoric acid:
PCl5 + 4H2O → H3PO4 + 5HCl
Reaction with sulfur dioxide yields thionyl chloride and phosphoryl chloride:
PCl5 + SO2 → SOCl2 + POCl3
Reaction with liquid hydrogen sulfide forms thiophosphoryl chloride, PSCl3:
PCl5 + H2S → PSCl3 + 2HCl
Phosphorus pentachoride converts arsenic to arsenic trichloride:
3PCl5 + 2As → 3AsCl3 + 3PCl3
Reaction with oxalic acid or boric acid yields phosphoryl chloride:
PCl5 + (COOH)2 → POCl3 + CO + CO2 + 2HCl
3PCl5 + 2B(OH)3 → 3POCl3 + B2O3 + 6HCl
Reaction with phosphorus pentoxide produces phosphoryl chloride:
3PCl5 + P2O5 → 5POCl3
Chemical Properties
Phosphorus pentachloride is a pale yellow, fuming solid with an odor like hydrochloric acid.162℃ sublimation and partial decomposition. All decomposed into chlorine gas and phosphorus trichloride at 300℃. Soluble in carbon disulfide, carbon tetrachloride. Decomposed in water, hydrolyzed in moist air into phosphoric acid and hydrogen chloride, white smoke and special irritating odor occur, strongly irritate the eyes.
Uses
Phosphorus pentachloride is used as a chlorinating agent to convert acids into acid chlorides, as a dehydrating agent, and as a catalyst.
Uses
As catalyst in manufacture of acetylcellulose; for replacing hydroxyl groups by Cl, particularly for converting acids into acid chlorides.
Definition
ChEBI: Phosphorus pentachloride is a phosphorus halide.
General Description
Phosphorus pentachloride is a greenish-yellow crystalline solid with an irritating odor. Phosphorus pentachloride is decomposed by water to form hydrochloric and phosphoric acid and heat. This heat may be sufficient to ignite surrounding combustible material. Phosphorus pentachloride is corrosive to metals and tissue. Long term exposure to low concentrations or short term exposure to high concentrations can result in adverse health effects from inhalation.
Reactivity Profile
Phosphorus pentachloride is a lightly yellow, fuming crystalline material, highly caustic, corrosive and toxic. Flammable by chemical reaction. Violent exothermic reaction with water or steam. When heated to decomposition Phosphorus pentachloride emits highly toxic fumes of chlorides and oxides of phosphorus. Explosive reaction with alkaline metals (sodium, potassium), urea. Ignites on contact with fluorine. Violent reaction with aluminum, chlorine trioxide, hydroxylamine, magnesium oxide, nitrobenzene, phosphorus(III) oxide, potassium. Carbamates form explosive products [Bretherick, 5th ed., 1995, p. 1360]. Reaction with the mixture of chlorine and chlorine dioxide causes explosion [Mellor, 1941, vol. 2, p. 281; 1940, vol. 8, p. 1013].
Health Hazard
Phosphorus pentachloride vapors are a strong irritant to the eyes and mucous membranes. Contact with skin can cause acid burns, as it reacts readily with moisture to form hydrochloric and phosphoric acids:
PCl5+4H2O→ H3PO4+5HCl
Chronic exposure to this compound can result in bronchitis.
LC50 value, inhalation (rats): 205 mg (24 ppm)/m3 (NIOSH 1986).
Flammability and Explosibility
Non flammable
Safety Profile
Poison by inhalation. Moderately toxic by ingestion. A severe eye, skin, and mucous membrane irritant. Corrosive to body tissues. Flammable by chemical reaction. Explosive reaction with chlorine dioxide + chlorine, sodium, urea + heat. Reacts to form explosive products with carbamates, 3'-methy-2-nitrobenzanilide (product explodes on contact with air). Ignites on contact with fluorine. Reacts violently with moisture, ClO3, hydroxyl- amine, magnesium oxide, nitrobenzene, phosphorus(Ⅲ) oxide, K. To fight fire, use CO2, dry chemical. Incompatible with aluminum, chlorine dioxide, chlorine,diphosphorus trioxide, fluorine, hydroxylamine, magnesium oxide, 3'-methyl- 2-nitrobenzanilide, nitrobenzene, sodium, urea, water. Will react with water or steam to produce heat and toxic and corrosive fumes. Used as a catalyst, chlorinating and dehydrating agent. When heated to decomposition it emits highly toxic fumes of Cland POx.
Potential Exposure
Phosphorus pentachloride is used as a as a chlorinating and dehydrating agent and as a catalyst. It is used in the manufacture of agricultural chemicals;chlorinated compounds; gasoline additives, plasticizers and surfactants; and in pharmaceutical manufacture
Shipping
UN1806 Phosphorus pentachloride, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
[All operations should be carried out in an efficient fume cupboard.] Sublime it at 160-170o in an atmosphere of chlorine. Excess chlorine is then displaced by dry N2 gas. All subsequent manipulations should be performed in a dry-box [Downs & Johnson J Am Chem Soc 77 2098 1955]. It fumes in moist air and attacks the eyes and the mucous membranes of the nose. It should not be breathed in and has very HARMFUL VAPOURS (wash burning eyes with aqueous NaHCO3).
Incompatibilities
Phosphorus pentachloride is a powerful oxidizer. Reacts with water (violent), magnesium oxide, chemically active metals, such as sodium and potassium, alkalis, amines, carbamates, aluminum powder, combustibles, fluorine, phosphorus pentoxide, phosphorus trioxide, and many other substances. Hydrolyzes in water (even in humid air) to form hydrochloric acid and phosphoric acid. Corrosive to many metals, forming flammable and explosive hydrogen gas. Attacks plastic and rubber.
Waste Disposal
Decompose with water, forming phosphoric and hydrochloric acids. Neutralize acids and dilute if necessary for discharge into the sewer system.
Phosphorus pentachloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5873 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5866 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Zjartschem | +86-571 87238903 | jocelynpan@zjarts.com | CHINA | 993 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | info@longyupharma.com | China | 2534 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| Hebei shuoxi biotechnology co. LTD | +8613081092107 | CHINA | 968 | 58 | |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
Related articles
- PCl5: Polarity, structure and properties
- Phosphorous pentachloride is an inorganic chemical compound with the molecular formula of PCl5. It mainly exists as a greenish....
- Dec 20,2023
View Lastest Price from Phosphorus pentachloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-19 | Phosphorus pentachloride
10026-13-8
|
US $10.70 / kg | 10kg | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-08-15 | Phosphorus pentachloride
10026-13-8
|
US $0.00 / kg | 1kg | 0.99 | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Phosphorus pentachloride
10026-13-8
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Phosphorus pentachloride
10026-13-8
- US $10.70 / kg
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Phosphorus pentachloride
10026-13-8
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Phosphorus pentachloride
10026-13-8
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
10026-13-8(Phosphorus pentachloride)Related Search:
1of4