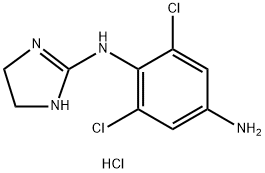
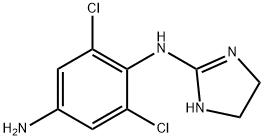
Apraclonidine hydrochloride
- CAS No.
- 73218-79-8
- Chemical Name:
- Apraclonidine hydrochloride
- Synonyms
- APRACLONIDINE HCL;iopidine;AL 02145;alo-2145;Iopidine UD;nc14hydrochloride;iopidineophthalmicsolution;APRACLONIDINE HYDROCHLORIDE;Apracloniding Hydrochloride;P-AMINOCLONIDINE HYDROCHLORIDE
- CBNumber:
- CB5439290
- Molecular Formula:
- C9H11Cl3N4
- Molecular Weight:
- 281.57
- MDL Number:
- MFCD00135922
- MOL File:
- 73218-79-8.mol
- MSDS File:
- SDS
| Melting point | >2300C |
|---|---|
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1.4 mg/mL Solutions may be stored for several days at 4°C |
| form | solid |
| color | white |
| Stability | Moisture Sensitive |
| CAS DataBase Reference | 73218-79-8(CAS DataBase Reference) |
| FDA UNII | D2VW67N38H |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H370 | |||||||||
| Precautionary statements | P301+P330+P331+P310-P308+P311 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 23/24/25 | |||||||||
| Safety Statements | 22-36/37/39-45 | |||||||||
| RIDADR | UN 2811 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2933290000 | |||||||||
| NFPA 704 |
|
Apraclonidine hydrochloride price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR3177 | Apraclonidine Hydrochloride pharmaceutical secondary standard, certified reference material | 73218-79-8 | 200MG | $534 | 2024-03-01 | Buy |
| Sigma-Aldrich | A0779 | p-Aminoclonidine hydrochloride solid | 73218-79-8 | 1MG | $92.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1041609 | Apraclonidine hydrochloride | 73218-79-8 | 100mg | $345.2 | 2024-03-01 | Buy |
| Cayman Chemical | 23904 | Apraclonidine (hydrochloride) ≥98% | 73218-79-8 | 1mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 23904 | Apraclonidine (hydrochloride) ≥98% | 73218-79-8 | 5mg | $161 | 2024-03-01 | Buy |
Apraclonidine hydrochloride Chemical Properties,Uses,Production
Chemical Properties
Crystalline Solid
Originator
Alfadrops,Cipla Limited,India
Uses
a-Adrenergic agonist; structural analog of clonidine. Used for treatment of post-surgical elevated intraocular pressure
Uses
Apraclonidine hydrochloride can be used as α-Adrenergic agonist; structural analog of clonidine and be used for treatment of post-surgical elevated intraocular pressure.
Definition
ChEBI: The hydrochloride salt of apraclonidine.
Manufacturing Process
The preparation of p-aminoclonidine (apraclonidine) consists of 6 steps.
In the first step 2,6-dichloro-4-nitroaniline was converted to 2,6-dicloro-4-
nitrophenylisothiocyanate by addition of thiophosgene in toluene according to
the method described in Great Britain Patent No.: 1,131,780 (Beck et al.).
The second step involved the conversation of 2,6-dichloro-4-nitrophenylisothiocyanate
to 1-(2-aminoethyl)-3-(2,6-dichloro-4-nitrophenyl)-thiourea
ethylenediamine solvate. The solution of 2,6-dicloro-4-nitrophenylisothiocyanate
(432 g, 1.73 mol) in 2 L of toluene was added dropwise to the
cooled (0°C) solution ethylenediamine (244 ml, 3.66 mol, 2.1 eq.) in toluene
(4 L) under a nitrogen atmosphere. 2-Propanol (1 L) was added and after 5
minutes, the solid was collected by filtration, washed with 20% 2-
propanol/toluene, and dried to a constant weight of 602 g (94%). This
product is hygroscopic, mp 120°C (dec.).
The third step was the conversation of 1-(2-aminoethyl)-3-(2,6-dichloro-4-
nitrophenyl)-thiourea ethylenediamine solvate to 2-[(2,6-dicloro-4-
nitrophenyl)imino]imidazoline ethylenediamine solvate. (500 g, 1.35 mol) of
above prepared thiourea solvate was suspended with toluene (4 L) and was
heated at reflux for 15 hours. The mixture was cooled to 23°C and 1 M
aqueous hydrochloric acid (4 L) was added. After stirring for 10 min the
biphasic mixture was filtered to remove a sticky insoluble material. The
aqueous phase was neutralized to pH=7.0 using 50% NaOH. After stirring for
1 hour the yellow solid was collected by filtration, washed with water (4 L)
and t-butyl methyl ether (2 L) and dried in air to constant weight of 195 g
(52%), m.p. 289-292°C.
The fourth step was the conversation of 2-[2,6-dichloro-4-nitrophenyl)
imino]imidazoline (150 g, 0.55 mol) in methanol (1,5 L) to 2-[(2,6-dichloro-4-
aminophenyl)imino]imidazoline by hydrogen with 30 g Raney nickel catalyst at
23°C for 22 hours. After removing the catalyst hydrogen chloride gas was
bubbled into solution until pH of the reaction mixture was 1.0. The solvent
was rotary removed in vacuum and the residual solid was slurried with 2-
propanol (1 L). The solvent was again removed by rotary evaporation, the
cream solid was triturated with 2-propanol (600 ml). After aging for 1 hour,
the solid was collected by filtration, washed with 2-propanol and t-butyl
methyl ether, and dried for 15 hours at 6°C and t-butyl methyl ether, and
dried for 15 hours at 60°C and 20 mm Hg. Yield of dihydrochloride 167 g (96%), mp 260°C (dec.).
The dihydrochloride was converted to the monochloride (step 5) by adding 5
M aqueous sodium hydroxide dropwise to pH=6.5 at 5°C for 2 hours. Yield of
hydrochloride 87%.
The last step was recrystallization of product from water. The recrystallized
material had m.p. 300°C. Calculated for: C9H10Cl2N4HCl: C, 38.39; H, 3.94;
N, 19.90; Cl, 37.78. Found: C, 38.36; H, 3.91; N, 19.83; Cl, 37.77.
brand name
Iopidine (Alcon).
Therapeutic Function
Antiglaucoma
Apraclonidine hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 | sales06@tgybio.com | China | 959 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15745 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
View Lastest Price from Apraclonidine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | Apraclonidine hydrochloride
73218-79-8
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-09-17 | Apraclonidine hydrochloride USP/EP/BP
73218-79-8
|
US $1.10 / g | 1g | 99.00% | 100 Tons min | Dideu Industries Group Limited |
-

- Apraclonidine hydrochloride
73218-79-8
- US $1.10 / g
- 99.0% min
- Shaanxi Dideu Medichem Co. Ltd
-

- Apraclonidine hydrochloride USP/EP/BP
73218-79-8
- US $1.10 / g
- 99.00%
- Dideu Industries Group Limited