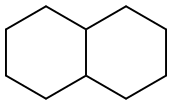
Decahydronaphthalene
- CAS No.
- 91-17-8
- Chemical Name:
- Decahydronaphthalene
- Synonyms
- DECALIN;DECALINE;NAPHTHANE;NAPHTHALANE;Decahydronaphthalin;Bicyclo[4.4.0]decane;DEKALIN;decalene;Dekalina;Naphthan
- CBNumber:
- CB6333241
- Molecular Formula:
- C10H18
- Molecular Weight:
- 138.25
- MDL Number:
- MFCD00004130
- MOL File:
- 91-17-8.mol
- MSDS File:
- SDS
| Melting point | −125 °C(lit.) |
|---|---|
| Boiling point | 189-191 °C(lit.) |
| Density | 0.896 g/mL at 25 °C(lit.) |
| vapor density | 4.76 (vs air) |
| vapor pressure | 42 mm Hg ( 92 °C) |
| refractive index |
n |
| Flash point | 57 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.006g/l (experimental) |
| form | Liquid |
| color | Clear |
| Odor | Aromatic, like turpentine; mild, characteristic. |
| explosive limit | 0.7-4.9%, 100°F |
| Water Solubility | 6 mg/L at 20 ºC |
| Sensitive | Hygroscopic |
| Merck | 14,2846 |
| BRN | 878165 |
| Henry's Law Constant | 7.00, 8.37, 10.6, 11.7, and 19.9 (x 10-2 atm?m3/mol) at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al., 1988) |
| Dielectric constant | 2.2(20℃) |
| Stability | Stable. Incompatible with oxidizing agents. Combustible. May form explosive peroxides. Heat and light accelerate peroxide formation. |
| InChIKey | NNBZCPXTIHJBJL-UHFFFAOYSA-N |
| LogP | 4.2 |
| Indirect Additives used in Food Contact Substances | DECAHYDRONAPHTHALENE |
| CAS DataBase Reference | 91-17-8(CAS DataBase Reference) |
| FDA UNII | 88451Q4XYF |
| NIST Chemistry Reference | Naphthalene, decahydro-(91-17-8) |
| EPA Substance Registry System | Decahydronaphthalene (91-17-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS02,GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H304-H314-H331-H410 | |||||||||
| Precautionary statements | P210-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,N,Xi | |||||||||
| Risk Statements | 20-34-51/53-36/37/38-65 | |||||||||
| Safety Statements | 26-36/37/39-45-60-24/25-23-62-61 | |||||||||
| RIDADR | UN 1147 3/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | QJ3150000 | |||||||||
| Autoignition Temperature | 482 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29021990 | |||||||||
| Toxicity | LD50 orally in rats: 4.2 g/kg; LC (in air) in rats: 500 ppm (Smyth) | |||||||||
| NFPA 704 |
|
Decahydronaphthalene price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D251 | Decahydronaphthalene, mixture of cis + trans reagent grade, 98% | 91-17-8 | 18L | $1240 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03101 | Decahydronaphthalene (mixture of cis-and trans isomers) for synthesis | 91-17-8 | 100mL | $36 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03101 | Decahydronaphthalene (mixture of cis-and trans isomers) for synthesis | 91-17-8 | 1L | $79.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03101 | Decahydronaphthalene (mixture of cis-and trans isomers) for synthesis | 91-17-8 | 25kg | $905 | 2024-03-01 | Buy |
| Sigma-Aldrich | 294772 | Decahydronaphthalene, mixture of cis + trans anhydrous, ≥99% | 91-17-8 | 1l | $251 | 2024-03-01 | Buy |
Decahydronaphthalene Chemical Properties,Uses,Production
Description
Decahydronaphthalene also referred to as Decalin is an organic compound that dissolves rubber, resins, waxes, fats, and oils. It is a colorless, aromatic hydrocarbon which is used as an alternative to turpentine, as a cleaning fluid and as a stain remover. Decahydronaphthalene was also used as a varnish remover for oil-based paintings in the past.
Chemical and Physical Properties
Decahydronaphthalene has a molecular weight of 138.254 g/mol, a monoisotopic mass of 138.141 g/mol and an exact mass of 138.141 g/mol. It has a heavy atom count of 10 and a complexity of 80.6. It is a clear, colorless liquid with a characteristic odor that resembles that of methanol.
Decahydronaphthalene has a flash point of 134°F and it is less dense than water. It indicates poor solubility in water at 250℃ and its vapours are denser than air. It has a density of 0.89 at 68°F220℃.
Decalin is very soluble in chloroform, ether, methanol and alcohol, and it is miscible with isopropyl alcohol, propyl, esters and a majority of the ketones. Decalin has a boiling point of 383/ 155.50℃ at 760 mm Hg and a melting point of -44°F -40°C.
After long periods of exposure, Decalin forms toxic concentrations of peroxides. When Decalin is heated to decomposition, it released acrid fumes and smoke.
Preparation
Decahydronaphthalene is prepared by the hydrogenation of tetralin at low pressure with rhodium as the catalyst. Decalin can be oxidized to yield a significant amount of the hydroperoxide derivative. The cis isomer of Decahydronaphthalene can be oxidized at a higher rate than the combination of the trans and cis isomers, or the trans isomer solely. Therefore, when Decahydronaphthalene is synthesized through the hydrogenation of an unsaturated homolog such as 1,2,3,4-tetrahydronaphthalene for application in the synthesis of hydroperoxide, the resulting product is made of the cis isomer primarily. This method results in Decahydronaphthalene that is saturated with the cis isomer. Such high-pressure processes are technical to execute, and they are relatively expensive. A low-pressure preparation process that entails the hydrogenation of tetralin to obtain cis Decahydronaphthalene is yet to be developed.
The rhodium catalyst is maintained on an inert support such as carbon or alumina, and the reaction is best conducted in the presence of a solvent, which may include a lower saturated carboxylic acid such as acetic acid. Other solvents such as propionic acid, mineral acids and non-acid solvents which may consist of water, hydrocarbons, ethers, esters, amines, amides and alcohol can also be used. The portion of the solvent should be one in which the reaction apparatus can withstand, while in some instances it may be best to omit the solvent. Acetic acid yields a higher rate of the cis isomer when it is applied as the solvent as opposed to the application of alumina as the support substance for the catalyst. The rhodium catalyst is applied as the hydrogenation catalyst, and it is best prepared through reduction of rhodium salts, which may include oxide or chloride. The progress of the hydrogenation reaction may depend on the quantity of the rhodium catalyst applied to the reaction. The tetralin to rhodium ratio is also dependent on the positioning of rhodium with an inert support, the configuration of the support and the apparatus used during the hydrogenation process. For instance, if the rhodium catalyst on an inert support such as carbon or alumina is about 5%, the reaction will yield satisfactory results. Furthermore, approximately 12-25g of the catalyst per mole of the Tetralin may result in satisfactory results. The optimum temperatures for this reaction are 50 C but the rate of the reaction may increase if the temperature is increased gradually from approximately 200 C250 C. The main advantage of this method is the fact that it can be conducted under low pressures of hydrogen, which may be as low as 0.5 atmospheres. Based on the above catalyst concentrations and reaction conditions, the total conversion of tetralin to Decahydronaphthalene may be obtained in about 1-2 hours. This reaction also yields the cis isomer which could be more than 90% of the product, which is relative to the total hydrogenation process which may be ascertained by the absorption of the theoretical amount of hydrogen by tetralin. To attain the desired cis Decahydronaphthalene, the reaction contents should be passed through a conventional fractional distillation to remove any trans isomers. It is fundamental to exercise care while conducting the distillation process using a basic packed column to yield a chromatographically pure cis Decahydronaphthalene.
Hazard Statements
Decalin is a flammable liquid, and it may also cause acute toxicity upon inhalation. It may cause skin irritation/corrosion upon contact as well as severe eye irritation/damage.
Chemical Properties
colourless liquid
Physical properties
Clear, colorless, flammable liquid with a mild methanol or hydrocarbon-like odor
Uses
Solvent for naphthalene, fats, resins, oils; alternate for turpentine in lacquers, shoe polishes, and waxes; component in motor fuels and lubricants
Uses
Solvent for naphthalene, waxes, fats, oils, resins, rubbers; motor fuel and lubricants; cleaning machinery; substitute for turpentine; shoe-creams; stain remover.
Uses
Decahydronaphthalene is widely used as an industrial solvent for resins and fuel additives. It is a substitute for turpentine in lacquers, shoe polishes and waxes.
Production Methods
Decalin occurs naturally in crude oil and is produced commercially by the catalytic hydrogenation of naphthalene. It is also a product of combustion and is released from natural fires.
Definition
ChEBI: An ortho-fused bicyclic hydrocarbon that is the decahydro- derivative of naphthalene.
General Description
A clear colorless liquid with an aromatic odor. Flash point 134°F. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Saturated aliphatic hydrocarbons, such as Decahydronaphthalene, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154].
Health Hazard
Inhalation or ingestion irritates nose and throat, causes numbness, headache, vomiting; urine may become blue. Irritates eyes. Liquid de-fats skin and causes cracking and secondary infection; eczema may develop.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by inhalation and ingestion. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Mildly toxic by skin contact. Human systemic effects by inhalation: conjunctiva irritation, unspecified olfactory and pulmonary system changes. Can cause kidney damage. Mutation data reported. A skin and eye irritant. Flammable liquid when exposed to heat or flame, can react with oxidzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes.
Environmental Fate
Photolytic. The following rate constants were reported for the reaction of decahydronaphthalene
and OH radicals in the atmosphere: 1.96 x 10-11 and 2.02 x 10-11 cm3/molecule?sec at 299 K for cis
and trans isomers, respectively (Atkinson, 1985). A photooxidation reaction rate constant of 2.00
x 10-11 was reported for the reaction of decahydronaphthalene (mixed isomers) and OH radicals in
the atmosphere at 298 K (Atkinson, 1990).
Chemical/Physical. Decahydronaphthalene will not hydrolyze because it has no hydrolyzable
functional group.
Purification Methods
Then the organic phase is separated, washed with water, saturated aqueous Na2CO3, again with water, dried with CaSO4 or CaH2 (and perhaps dried further with Na), filtered and distilled under reduced pressure (b 63-70o/10mm). It has also been purified by repeated passage through long columns of silica gel previously activated at 200-250o, followed by distillation from LiAlH4 and storage under N2. Type 4A molecular sieves can be used as a drying agent. Storage over silica gel removes water and other polar substances. [For the separation of cis and trans isomers see Seyer & Walker J Am Chem Soc 60 2125 1938, and Baker & Schuetz J Am Chem Soc 69 1250 1949.]
Decahydronaphthalene Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 | sales@richfortunechem.com | China | 303 | 57 |
| Hong Kong Excellence Biotechnology Co., Ltd. | +86-86-18838029171 +8618126314766 | ada@sh-teruiop.com | China | 893 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
View Lastest Price from Decahydronaphthalene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-25 | Decahydronaphthalene
91-17-8
|
US $100.00 / kg | 1kg | >99% | 50 TONS | Hong Kong Excellence Biotechnology Co., Ltd. | |
 |
2023-07-27 | Decahydronaphthalene
91-17-8
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2019-07-10 | Decahydronaphthalene
91-17-8
|
US $1.00 / ASSAYS | 1ASSAYS | 98% | 1kg,2kg,100kg | Career Henan Chemical Co |
-

- Decahydronaphthalene
91-17-8
- US $100.00 / kg
- >99%
- Hong Kong Excellence Biotechnology Co., Ltd.
-

- Decahydronaphthalene
91-17-8
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- Decahydronaphthalene
91-17-8
- US $1.00 / ASSAYS
- 98%
- Career Henan Chemical Co
91-17-8(Decahydronaphthalene)Related Search:
1of4