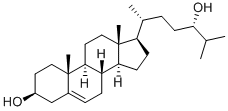
(3β,24S)-Cholest-5-ene-3,24-diol
- CAS No.
- 474-73-7
- Chemical Name:
- (3β,24S)-Cholest-5-ene-3,24-diol
- Synonyms
- 24-hydroxycholesterol;24(S)-HYDROXYCHOLESTEROL;5-CHOLESTEN-3β, 24(S)-DIOL;cholest-5-ene-3β,24(S)-diol;(24S)-24-hydroxycholesterol;cholest-5-ene-3-beta,24-diol;(3β,24S)-Cholest-5-ene-3,24-diol;(3β,24S)-Cholest-5-ene-3,24-diol;Cholest-5-ene-3,24-diol, (3β,24S)-;24(S)-hydroxycholesterol (24(S)-HC)
- CBNumber:
- CB6498177
- Molecular Formula:
- C27H46O2
- Molecular Weight:
- 402.65
- MDL Number:
- MFCD03695560
- MOL File:
- 474-73-7.mol
- MSDS File:
- SDS
| Melting point | 174-176 °C |
|---|---|
| Boiling point | 513.1±23.0 °C(Predicted) |
| Density | 1.03±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 10 mg/ml) or in Ethanol (up to 10 mg/ml). |
| form | powder |
| pka | 15.03±0.70(Predicted) |
| color | white to beige |
| optical activity | [α]/D -45 to -52°, c = 1 in chloroform-d |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| FDA UNII | 47IMW63S3F |
(3β,24S)-Cholest-5-ene-3,24-diol price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1648 | 24(S)-Hydroxycholesterol ≥98% (HPLC) | 474-73-7 | 1MG | $124 | 2023-06-20 | Buy |
| Sigma-Aldrich | SML1648 | 24(S)-Hydroxycholesterol ≥98% (HPLC) | 474-73-7 | 5MG | $504 | 2023-06-20 | Buy |
| Cayman Chemical | 10009931 | 24(S)-hydroxy Cholesterol ≥95% | 474-73-7 | 1mg | $68 | 2024-03-01 | Buy |
| Cayman Chemical | 10009931 | 24(S)-hydroxy Cholesterol ≥95% | 474-73-7 | 5mg | $319 | 2024-03-01 | Buy |
| Cayman Chemical | 10009931 | 24(S)-hydroxy Cholesterol ≥95% | 474-73-7 | 10mg | $601 | 2024-03-01 | Buy |
(3β,24S)-Cholest-5-ene-3,24-diol Chemical Properties,Uses,Production
Description
24(S)-
Uses
(3β,24S)-Cholest-5-ene-3,24-diol is used as a biomarker in the analysis of disease.
Uses
(3β,24S)-Cholest-5-ene-3,24-diol is used as a biomarker in the analysis of disease.
Definition
ChEBI: (24S)-24-hydroxycholesterol is a 24-hydroxycholesterol that has S configuration at position 24. It is the major metabolic breakdown product of cholesterol in the brain. It has a role as a mouse metabolite, a biomarker and a human blood serum metabolite.
General Description
24(S)-hydroxycholesterol (24HC) is synthesized from cholesterol in brain dendrites by the action of enzyme cholesterol 24-hydroxylase (CYP46A1). It is catabolized to bile acids in the liver.
Biochem/physiol Actions
24(S)-hydroxycholesterol (24HC) elevated levels are reported in liver inflammation and fibrosis. It is a N-methyl-D-aspartate receptor (NMDAR) modulator. The levels of 24HC are potential indicators of brain development as well as pathology including Alzheimer′s disease (AD) and multiple sclerosis. Polymorphism in the cholesterol 24-hydroxylase (CYP46A1) gene leads to elevated 24HC levels and toxicity. 24HC is a mediator of apoptosis and necroptosis. Elevated levels of 24HC are reported in liver inflammation and fibrosis.
References
1) Lujohann et al. (1996), Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into circulation; Proc. Natl. Acad. Sci. USA, 93 9799 2) Chawla et al. (2001), Nuclear receptors and lipid physiology:opening the X-files; Science, 294 1866 3) Kolsch et al. (1999), The neurotoxic effect of 24-hydroxycholesterol on SH-SY5Y human neuroblastoma cells; Brain Res., 818 171 4) Yamanaka et al. (2011), 24(S)-hydroxycholesterol induces neuronal cell death through necroptosis, a form of programmed necrosis; J. Biol. Chem., 286 24666 5) Wang et al. (2010), A second class of nuclear receptors for oxysterols: regulation of RORalpha and RORgamma activity by 24(S)-hydroxycholesteraol (cerebrosterol); Biochim. Biophys. Acta, 1801 917 6) Leoni and Caccia (2013), Potential diagnostic applications of side chain oxysterols analysis in plasma and cerebrospinal fluid; Biochem. Pharmacol., 86 26 7) Urano et al. (2013), Suppression of amyloid-β production by 24S-hydroxycholesterol via inhibition of intracellular amyloid precursor protein trafficking; FASEB J.,?27?4305
(3β,24S)-Cholest-5-ene-3,24-diol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Labnetwork lnc. | +86-27-50766799 +8618062016861 | contact@labnetwork.com | China | 19994 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4863 | 58 |
| Guangzhou Isun Pharmaceutical Co., Ltd | 020-39119399 18927568969 | isunpharm@qq.com | China | 4428 | 55 |
| Shanghai TaoSu Biochemical Technology Co., Ltd. | 021-33632979 | info@tsbiochem.com | China | 8073 | 58 |
| Sichuan Wei Keqi Biological Technology Co., Ltd. | 028-81700200 18116577057 | 3003855609@qq.com | China | 7892 | 56 |