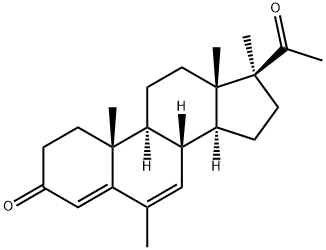
Medrogestone
- CAS No.
- 977-79-7
- Chemical Name:
- Medrogestone
- Synonyms
- MEDROGESTONE;MEDROGESTONE F·S;MEDROGESTONE 977-79-7;Medrogestone USP/EP/BP;6,17α-methyl-4,6-pregnadien-3,20-dione;4,6-PREGNADIEN-6,17-DIMETHYL-3,20-DIONE;6,17-dimethyl-pregna-4,6-diene-3,20-dione;Pregna-4,6-diene-3,20-dione, 6,17-dimethyl-
- CBNumber:
- CB7181100
- Molecular Formula:
- C23H32O2
- Molecular Weight:
- 340.5
- MDL Number:
- MFCD00271888
- MOL File:
- 977-79-7.mol
- MSDS File:
- SDS
| Melting point | 144-146° |
|---|---|
| alpha | D23 +79° (c = 1 in chloroform) |
| Boiling point | 416.28°C (rough estimate) |
| Density | 1.0555 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| Water Solubility | 1.82mg/L(25 ºC) |
| CAS DataBase Reference | 977-79-7(CAS DataBase Reference) |
| FDA UNII | 077DN93G5B |
| ATC code | G03DB03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 |
Medrogestone Chemical Properties,Uses,Production
Originator
Colpro,Ayerst,Italy,1970
Uses
Progestin.
Definition
ChEBI: Medrogestone is a corticosteroid hormone.
Manufacturing Process
The manufacturing process as described in US Patent 3,170,936 uses the readily available methyl 3β-hydroxy-17α-methyl-δ5-etienate (I), described by Plattner in Helv. Chim. Acta, vol. 31, p 603 (1948), as the starting material. The etienic acid ester (I) may also be called 17α-methyl-17βcarbomethoxyandrost-5-ene-3β-ol.
3β,5α,6β-Trihydroxy-17α-Methyl-17β-Carbomethoxyandrostane (II): 5 g of
17α-Methyl-17β-carbomethoxyandrost-5-ene-3β-ol (I) is dissolved in formic
acid (50 ml) and heated on the steam bath for 10 minutes. The solution is
cooled to room temperature and a crystalline solid precipitates. This is stirred,
30% hydrogen peroxide (5 ml) is added, and the reaction mixture is left at
room temperature for 2 hours. The clear solution is poured into water 1300
ml) and the solid which precipitates is filtered.
It is dissolved in hot methanol and heated on the steam bath with 10%
methanolic potassium hydroxide solution (15.8 ml) for 10 minutes. Then more
potassium hydroxide solution (2 ml) is added, the solution is cooled and on
dilution with water a solid (II), MP 245° to 255°C, is obtained. A second crop
is obtained from the mother liquors. Several recrystallizations from acetone
yield an analytical sample, MP 262° to 265°C, [α]D24 is -2.1°.
3β-Acetoxy-5α-Hydroxy-17α-Methyl-17β-Carbomethoxyandrostane-6-one
(IIIb): 3β,5α,6βp-Trihydroxy-17α-methyl-17β-carbomethoxyandrostane (II, 5.2
g) is dissolved in methanol (105 ml) to which ether (105 ml) and water (84
ml) are added. Then N-bromosuccinimide (5.2 g) is added with stirring and
the clear solution is left in the refrigerator for 3 hours. The ether is removed
under reduced pressure at room temperature and a crystalline solid (IIIa)
separates, MP 268° to 272°C.
The above substance is dissolved in pyridine (15 ml) and acetic anhydride
(7.5 ml), and heated on the steam bath for ? hour. The product (IIIb)
crystallizes from aqueous ethanol in leaflets, MP 237° to 239°C. An analytical
sample has MP 241° to 243°C.
3β,5α,6β-Trihydroxy-6α,17α-Dimethyl-17β-Carbomethoxyandrostane (IV): 3βAcetoxy-5α-hydroxy-17α-methyl-17β-carbomethoxyandrostan-6-one (III,
1.004 g) is dissolved in dry benzene (25 ml) and methyl magnesium bromide
solution in ether (3 M, 10 ml) is added. The reaction mixture is diluted with
dry tetrahydrofuran (25 ml) and allowed to stand at room temperature for 20
hours, Excess Grignard reagent is quenched by adding a saturated solution of
ammonium chloride. The organic layer is separated and the aqueous layer is
extracted with ethyl acetate.
After washing the combined extracts with ammonium chloride solution and
water and working up in the usual way a white solid (IV) is obtained which
after one recrystallization from aqueous methanol has MP 242° to 243°C. The
infrared spectrum of this compound indicates the presence of a carbomethoxy
group (1,730 cm-1) and disappearance of the 6-keto group together with the
presence of an ester group (1,727 cm-1). This substance is used without
further purification for the next step.
3β,5α,6β-Trihydroxy-6α,17α-Dimethylpregnan-20-one (V): Crude 3β,5α,6βtrihydroxy-6α,17α-dimethyl-17β-carbomethoxyandrostane (IV, 773 mg) is
dissolved in dry benzene (25 ml) and tetrahydrofuran (freshly distilled over
lithium aluminum hydride, 25 ml). To the stirred solution under dry N2 there is
added methyl magnesium bromide solution in ether (3 M, 10 ml) over a
period of 10 minutes. Then the ether and tetrahydrofuran are almost all
distilled and the resulting solution is refluxed for 3 hours (solid precipitates
during the reaction). The reaction mixture is cooled and worked up in the
same way as in the previous experiment leaving a white solid (V) with an
infrared spectrum which indicates the presence of a 20-ketone group (1,690
cm-1), a sample of which is recrystallized to MP 238° to 240°C.
Analysis confirmed the empirical formula C23H38O4H2O: Required: C, 69.60%;
H, 10.17%. Found: C, 69.90%; H, 10.15%.
Alternatively, 25.0 g of either 3β,5α-dihydroxy-17α-methy-17βcarbomethoxyandrostan-6-one (IIIa) or 25.0 g of its 3β-acetate (IIIb), are
dissolved in dry tetrahydrofuran (1,250 ml, freshly distilled over lithium
aluminum hydride) and dry benzene (2,000 ml) is added. Methyl magnesium
bromide in ether solution (3 M, 750 ml) is added to the stirred solution and
the resulting mixture is stirred at room temperature for 16 hours. An
additional quantity of methyl magnesium bromide solution in ether (2 M, 375
ml) is added, and 1,250 ml of the solvent mixture are distilled off. The
resulting mixture is refluxed for 5 hours and worked up as described above,
yielding compound (V) as a colorless oil.
5α,6β-Dihydroxy-6α,17α-Dimethylpregnane-3,20-dione (VI): Crude 3β,5α,6βtrihydroxy-6α,17α-Dimethylpregnan-20-one (V, 650 mg) is dissolved in
acetone (freshly distilled over potassium permanganate, 150 ml) and cooled in
an ice-water bath with stirring. Then excess chromic acid solution (8 N) is
added and stirring is continued at room temperature for 4 minutes. The
reaction mixture is poured into water and extracted with ethyl acetate. The
combined extracts are washed with dilute sodium bicarbonate solution and
water and then dried over magnesium sulfate. Removal of the solvent leaves a
white solid (VI). This crude product is used for the next step. Its IR spectrum
shows a strong band at 1,705 cm-1. A sample is recrystallized to MP 243° to
245°C (dec.).
6,17α-Dimethyl-4,6-Pregnadiene-3,20-dione (VII): 5α,6β-Dihydroxy-6α,17αdimethylpregnane-3,20-dione (VI, 553 mg) is dissolved in absolute ethanol
(60 ml) and two drops of concentrated hydrochloric acid are added. This
solution is heated on a steam bath for 45 minutes, cooled, diluted with water
and extracted with ether. The combined extracts are washed with dilute
sodium bicarbonate solution and water and subsequently dried over
magnesium sulfate. After the solvent has been removed a syrup remains and
the UV spectrum of this substance indicates the presence of a δ4,6-
ketone.Elution of this material over alumina (Woelm, Grade III, 25 g) with 1:1
hexane-benzene gives a crystalline substance, MP 138° to 141°C which, after
one recrystallization from ether, has an infrared spectrum identical to that of
an authentic sample of 6,17α-dimethyl-4,6-pregnadiene-3,20-dione (VII).
Therapeutic Function
Progestin
Medrogestone Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8348 | 58 |
| Nanjing Shizhou Biotechnology Co., Ltd | +86-15850508050 +86-15850508050 | sean.lv@synzest.com | China | 323 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12470 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hebei Bonster Technology Co.,Limited | +8613315996897 | bsterltd.wendy@gmail.com | China | 796 | 58 |
| Longyan Tianhua Biological Technology Co., Ltd | 0086 18039857276 18039857276 | CHINA | 2783 | 58 | |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
View Lastest Price from Medrogestone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | Medrogestone
977-79-7
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd | |
 |
2023-01-31 | Medrogestone
977-79-7
|
US $0.00 / Kg/Bag | 1Kg/Bag | 99% | 20ton | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-10-19 | Medrogestone
977-79-7
|
US $0.00 / g | 1g | 99.9% | 200kgs | Hebei Bonster Technology Co.,Limited |
-

- Medrogestone
977-79-7
- US $1.10 / g
- 99.0% min
- Shaanxi Dideu Medichem Co. Ltd
-

- Medrogestone
977-79-7
- US $0.00 / Kg/Bag
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Medrogestone
977-79-7
- US $0.00 / g
- 99.9%
- Hebei Bonster Technology Co.,Limited