Papaverine hydrochloride
- CAS No.
- 61-25-6
- Chemical Name:
- Papaverine hydrochloride
- Synonyms
- PAPAVERINE HCL;paph;dipav;BOC-azide;1-(3,4-DIMETHOXYBENZYL)-6,7-DIMETHOXYISOQUINOLINE HYDROCHLORIDE;lapav;vasal;dynovas;optenyl;pameion
- CBNumber:
- CB7689772
- Molecular Formula:
- C20H22ClNO4
- Molecular Weight:
- 375.85
- MDL Number:
- MFCD00012745
- MOL File:
- 61-25-6.mol
| Melting point | 226°C (dec.) |
|---|---|
| storage temp. | Amber Vial, Refrigerator |
| solubility | H2O: 25 mg/mL |
| form | powder |
| color | white |
| PH | pH (20g/l, 25℃) : 3.0~4.0 |
| Water Solubility | freely soluble |
| Sensitive | Light Sensitive |
| Merck | 14,7019 |
| BRN | 3921435 |
| Stability | Stable, but may be light sensitive. |
| CAS DataBase Reference | 61-25-6(CAS DataBase Reference) |
| FDA UNII | 23473EC6BQ |
| NCI Drug Dictionary | Cerespan |
| EPA Substance Registry System | Papaverine hydrochloride (61-25-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P405-P501 | |||||||||
| Hazard Codes | Xn,C,F | |||||||||
| Risk Statements | 22-34-11 | |||||||||
| Safety Statements | 22-45-36/37/39-26-16 | |||||||||
| RIDADR | UN 1544 6.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NW8575000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29391900 | |||||||||
| Toxicity | LD50 in mice, rats (mg/kg): 27.5, 20 i.v.; 150, 370 s.c. (Levis) | |||||||||
| NFPA 704 |
|
Papaverine hydrochloride price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P3510 | Papaverine hydrochloride powder | 61-25-6 | 5g | $51.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1496008 | Papaverine hydrochloride United States Pharmacopeia (USP) Reference Standard | 61-25-6 | 200mg | $436 | 2024-03-01 | Buy |
| Alfa Aesar | B25412 | Papaverine hydrochloride, 99% | 61-25-6 | 5g | $47.3 | 2024-03-01 | Buy |
| Alfa Aesar | B25412 | Papaverine hydrochloride, 99% | 61-25-6 | 25g | $125 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR1182 | Papaverine hydrochloride Pharmaceutical Secondary Standard; Certified Reference Material | 61-25-6 | 500mg | $180 | 2024-03-01 | Buy |
Papaverine hydrochloride Chemical Properties,Uses,Production
Description
Papaverine Hydrochloride, USP, is the hydrochloride of an alkaloid obtained from opium or prepared synthetically. It belongs to the benzylisoquinoline group of alkaloids. It does not contain a phenanthrene group as do morphine and codeine.
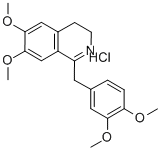
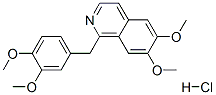
Papaverine Hydrochloride, USP, is 6,7-dimethoxy-1-veratrylisoquinoline hydrochloride and contains, on the dried basis, not less than 98.5% of CHNO.HCI. The molecular weight is 375.85. The structural formula is as shown.
Papaverine Hydrochloride occurs as white crystals or white crystalline powder. One gram dissolves in about 30 mL of water and in 120 mL of alcohol. It is soluble in chloroform and practically insoluble in ether.
Papaverine Hydrochloride Injection, USP, is a clear, colorless to pale-yellow solution.
Papaverine Hydrochloride, for parenteral administration, is a smooth-muscle relaxant that is available in vials containing 30 mg/mL. Each vial also contains edetate disodium 0.005%. The 10 mL vials also contain chlorobutanol 0.5% as a preservative. pH may be adjusted with sodium citrate and/or citric acid.
Chemical Properties
PAPAVERINE HYDROCHLORIDE is Crystalline Solid
Physical properties
Appearance: colorless prismatic or acicular crystal. Melting point: 147–148? °C. Relative density: 1.337 (20/4?°C). Solubility: very soluble in benzene, acetone, hot ethanol, and glacial acetic acid; soluble in concentrated sulfuric acid; slightly soluble in ether, chloroform, and carbon tetrachloride; and insoluble in water. The common crystalline salt is hydrochloride salt, papaverine hydrochloride.
History
Human beings have a long history of using opium poppy as food and narcotics,
which can be dated back to the Neolithic age. The opium, which is the dried product
of the opium poppy juice, is widely used as analgesics in the old and new civilizations of the old continent except the Chinese civilization.
In 1848, German chemist Georg Merck successfully isolated a new alkaloid from
the opium liquor for the first time and named it as “papaverine” . He also determined the correct molecular formula of papaverine as C20 H21NO4 and prepared the
papaverine hydrochloride and nitrate by recrystallization method. Then,
Goldschmiedt et?al. put forward the molecular structure of papaverine by studying
its oxidation products in 1883 and determined the isoquinoline ring as the core
structure of papaverine in 1888 .
Since the content of papaverine in plants is very low (less than 1%) and can
hardly meet the clinical needs, so Pictet and Gams proposed a synthetic method of
papaverine in 1909, which makes the large-scale industrialized production of papaverine possible .
Study on the pharmacology of papaverine was first published in 1914 by
Professor Pal of Vienna, who found that papaverine possessed smooth muscle relaxation effect without causing smooth muscle paralysis and can be used for the treatment of hypertension, angina, and acute uremia .
Uses
Papaverine hydrochloride is as effective as sodium diclofenac for the short-term relief of acute renal colic pain and may be advantageous in patients with contraindications for nonsteroidal anti-inflammatory drugs. However, sodium diclofenac appears to provide a longer effective analgesia.
Papaverine hydrochloride belongs to the group of benzylisoquinoline alkaloid. It is obtained from opium poppy.
Papaverine hydrochloride has been used:
in oxygenated Krebs solution for tissue mechanics analysis
as a vasodilator in heparinized horse serum in tissue uptake technique
as an inhibitor of H-MPP+ uptake into stably transfected 293 cells expressing either extraneuronal monoamine transporter (EMT) human or EMT rat
Uses
Anti-spasmodic, The treatment of erectile dysfunction
Uses
Smooth muscle relaxant found in opium. Vasodilator (cerebral)
Indications
Papaverine is mainly used for the treatment of ischemia caused by cerebral, cardiac, and peripheral vascular spasm, as well as visceral spasm of the kidney, gallbladder, or gastrointestinal tract.
brand name
Pavabid (Hoechst Marion Roussel).
General Description
Papaverine hydrochloride,6,7-dimethoxy-1-veratrylisoquinoline hydrochloride,was isolated by Merck in 1848 from opium,in which it occurs to the extent of about 1%. Althoughits natural origin is closely related to morphine, the pharmacologicalactions of papaverine hydrochloride areunlike those of morphine. Its main effect is as a spasmolyticon smooth muscle, acting as a direct, nonspecificrelaxant on vascular, cardiac, and other smooth muscle.Because of its broad antispasmodic action on ACh muscarinicreceptors, it is often called a nonspecific antagonist.Papaverine hydrochloride has been used in the treatmentof peripheral vascular disorders, but its use is limitedby lack of potency.
Papaverine hydrochloride interferes with the mechanismof muscle contraction by inhibiting the cyclic nucleotidephosphodiesterases in smooth muscle cells responsible forconverting cAMP and cyclic guanosine monophosphate(cGMP) to 5' -AMP and 5' -GMP, respectively. The increasedlevels of cAMP and cGMP are associated with muscle relaxation through their phosphorylation of myosinlight-chain kinase.
General Description
White powder. pH (0.05 molar solution) 3.9. pH (2% aqueous solution) 3.3.
Air & Water Reactions
Water soluble.
Reactivity Profile
Papaverine hydrochloride is sensitive to light. .
Health Hazard
SYMPTOMS: Symptoms of exposure to Papaverine hydrochloride may include sleepiness, slowed respiration and slowed heart beat.
Fire Hazard
Flash point data for Papaverine hydrochloride are not available, but Papaverine hydrochloride is probably combustible.
Biochem/physiol Actions
Papaverine is originally used to resolve male impotence.
Pharmacology
As a vasodilator, papaverine is a nonspecific antispasmodic drug of smooth muscle, which is especially effective on pulmonary artery, coronary artery, and great vessels, producing systemic and nonspecific hypotensive and smooth muscle relaxation effect. Papaverine has a direct effect on the smooth muscle cells by inhibiting phosphodiesterase, thus increasing the intracellular concentration of cyclic adenosine monophosphate (cAMP), which further removes the catalase calcium from cytoplasmic of vascular smooth muscle, leading to the direct smooth muscle relaxation without nerve. Papaverine can also inhibit the cardiac conduction, directly act on myocardial cells, and prolong the refractory period. No effect of papaverine on the central nervous system has been found. High dose of papaverine can cause hypotension and tachycardia.
Clinical Use
Papaverine hydrochloride is used for the treatment of ischemia induced by brain, heart, and peripheral vascular spasm, as well as visceral spasm of the kidney, bladder, or gastrointestinal tract. Besides, papaverine is also suitable for angina and arterial embolism, occasionally used for the treatment of erectile dysfunction. In recent years, papaverine hydrochloride also combines with other drugs like nimodipine to treat the vasospasm and crisis triggered by subarachnoid hemorrhage and calculi-induced acute renal colic. The main adverse effects of papaverine include liver function damage, exaggerated respiration induced by rapid parenteral administration, facial flushing, heart rate acceleration, as well as hypotension with vertigo. In addition, overdose of papaverine can lead to blurred vision, diplopia, lethargy, and weakness.
Safety Profile
Poison by ingestion, intraperitoneal, intraduodenal, intravenous, and subcutaneous routes. Human systemic effects: metabolic acidosis, pulse rate increase. An experimental teratogen. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and HCl. See also PAPAVERINE
Purification Methods
Recrystallise it from H2O. It sublimes at 140o/0.1mm. Its solubility in H2O is 5%. [Saunders & Srivastava J Pharm Pharmacol 3 78 1951, Biggs Trans Faraday Soc 50 800 1954.] The free base has m 148-150o, The picrate has m 186-189o(dec, 186-186.5o(dec) [Bobbitt J Org Chem 22 1729 1957]. [Beilstein 21 II 202, 21 III/IV 2788, 21/6 V 182.]
Papaverine hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29824 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Longyan Tianhua Biological Technology Co., Ltd | 0086 18039857276 18039857276 | CHINA | 2783 | 58 | |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 7121 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 8331 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
View Lastest Price from Papaverine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-16 | Papaverine hydrochloride
61-25-6
|
US $50.00 / KG | 1KG | >99% | 20tons | Weijer International Trade (Hebei) Co., Ltd | |
 |
2022-03-25 | Copavin
61-25-6
|
US $100.00 / bag | 1bag | 99 | 500 | Shanxi Naipu Import and Export Co.,Ltd | |
![Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-, hydrochloride (1:1) pictures](https://img.chemicalbook.com/ProductImageEN/2020-1/Small/8bc99468-0952-462c-b2a2-9c76546d4ce8.jpg) |
2020-01-10 | Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-, hydrochloride (1:1)
61-25-6
|
US $9.80 / KG | 1KG | ≥98% | 20 tons | Career Henan Chemical Co |
-

- Papaverine hydrochloride
61-25-6
- US $50.00 / KG
- >99%
- Weijer International Trade (Hebei) Co., Ltd
-

- Copavin
61-25-6
- US $100.00 / bag
- 99
- Shanxi Naipu Import and Export Co.,Ltd
-
![Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-, hydrochloride (1:1) pictures](https://img.chemicalbook.com/ProductImageEN/2020-1/Small/8bc99468-0952-462c-b2a2-9c76546d4ce8.jpg)
- Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-, hydrochloride (1:1)
61-25-6
- US $9.80 / KG
- ≥98%
- Career Henan Chemical Co





