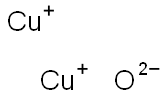Cuprous oxide
- CAS No.
- 1317-39-1
- Chemical Name:
- Cuprous oxide
- Synonyms
- COPPER OXIDE;COPPER(I) OXIDE;Cupper oxide;dicopper oxide;Copper oxide (Cu2O);Copox;Cu2-O;Cuprox;Nordox;Perecot
- CBNumber:
- CB9853041
- Molecular Formula:
- Cu2O
- Molecular Weight:
- 143.09
- MDL Number:
- MFCD00010974
- MOL File:
- 1317-39-1.mol
- MSDS File:
- SDS
| Melting point | 1232 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 1800 °C | ||||||||||||||
| Density | 6 g/mL at 25 °C (lit.) | ||||||||||||||
| refractive index | 2.705 | ||||||||||||||
| Flash point | 1800°C | ||||||||||||||
| storage temp. | Inert atmosphere,Room Temperature | ||||||||||||||
| solubility | insoluble in H2O | ||||||||||||||
| form | powder | ||||||||||||||
| color | red | ||||||||||||||
| Specific Gravity | 6. | ||||||||||||||
| Water Solubility | practically insoluble | ||||||||||||||
| Sensitive | Air & Moisture Sensitive | ||||||||||||||
| Crystal Structure | Cu2O type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Merck | 14,2664 | ||||||||||||||
| Space group | Pn3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
||||||||||||||
| Dielectric constant | 18.1(Ambient) | ||||||||||||||
| Stability | Stable. May be air or light sensitive. | ||||||||||||||
| CAS DataBase Reference | 1317-39-1(CAS DataBase Reference) | ||||||||||||||
| FDA UNII | T8BEA5064F | ||||||||||||||
| NIST Chemistry Reference | Copper(i) oxide(1317-39-1) | ||||||||||||||
| EPA Substance Registry System | Cuprous oxide (1317-39-1) | ||||||||||||||
| Pesticides Freedom of Information Act (FOIA) | Cuprous oxide |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H410 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P273-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,F | |||||||||
| Risk Statements | 22-50/53-51/53-11 | |||||||||
| Safety Statements | 22-60-61-16-7 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GL8050000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28255000 | |||||||||
| Toxicity | LD50 orally in rats: 0.47 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Cuprous oxide price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 208825 | Copper(I) oxide powder, ≤7 μm, 97% | 1317-39-1 | 25g | $64.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208825 | Copper(I) oxide powder, ≤7 μm, 97% | 1317-39-1 | 2kg | $382 | 2024-03-01 | Buy |
| Alfa Aesar | 012300 | Copper(I) oxide, 97% (Cu + Cu2O Assay) | 1317-39-1 | 500g | $49.8 | 2024-03-01 | Buy |
| Alfa Aesar | 012300 | Copper(I) oxide, 97% (Cu + Cu2O Assay) | 1317-39-1 | 2kg | $124 | 2023-06-20 | Buy |
| Strem Chemicals | 93-2944 | Copper(I) oxide, min. 95% | 1317-39-1 | 250g | $29 | 2024-03-01 | Buy |
Cuprous oxide Chemical Properties,Uses,Production
General description
cuprous oxide appears as dark red cubic crystal or orange-yellow crystalline powder, being toxic. It has a relative density of 6.04 with the melting point being 1235 ° C. When being heated to 1800 °C, it can undergo decomposition and release oxygen. Although it can be stably presented, it can be gradually oxidized into copper oxide in wet air. It is insoluble in water and ethanol, being soluble in dilute sulfuric acid and can subject to disproportionation into copper sulfate and metal copper. When dissolved in nitric acid, it can be oxidized into copper nitrate. It can be dissolved in concentrated hydrochloric acid or ammonia and respectively generate stable complex H3 [CuCl4] or colorless complex [Cu (NH3) 2] +. The latter one can be easily oxidized by air to blue [Cu (NH3) 4] 2+.
Cuprous oxide is presented in nature in the form of cuprite. It can be obtained through adding appropriate amount of hydrazine hydrate into the copper acetate solution or adding the reducing agent such as glucose to the alkaline solution (add sodium potassium tartrate or citrate to prevent the precipitation of copper hydroxide) of copper salt. According to the preparation method and particle size, cuprous oxide has different sizes including yellow, red or brown colors.
Copper oxide can be used as the coloring agent (red) for the manufacture of red glass, red porcelain glaze and ceramic, ship bottom paint, agricultural fungicides, organic synthesis catalyst, rectifier materials and bottom coatings, also used in electroplating industry and used as organic synthesis catalyst. Cuprous oxide can also be used as a reducing agent in the determination of nitrogen content in azo compounds.
The method for industrial preparation of cuprous oxide is through the calcination of precipitated copper powder and copper oxide mixture in sealed closed system.

cuprous oxide powder
Chemical properties
Cuprous oxide is a yellow to red crystalline powder. The color difference is caused by the size of the particles. The structure is red copper type structure (cubic crystal system). The relative density: 6.04; the melting point: 1232 °C with the heat of formation being 166.67 kJ/mol. In 1800 °C, it will lose oxygen, being insoluble in water and soluble in ammonia with the action with concentrated hydrochloric acid producing white cuprous chloride crystal powder.
Copper oxide contains excess amount of oxygen atoms with the appearance of holes of Cu + lattice that plays a role in receiving electrons. It is a P-type semiconductor. The energy level is about 1.5 eV with the accepting energy level being 0.3~0.5 eV above the valence band. In addition, it is well known that the spectrum of the absorption excitation atoms represents the configuration of hydrogen atoms.
It can absorb carbon dioxide at room temperature, but can be released upon being heated in the 60~70 °C. Copper oxide, when being reduced with hydrogen at 150 °C, can be converted to cuprous oxide; if there is oxygen, after appropriate heating (200 °C), cuprous oxide can return back to copper oxide, taking this property, we can remove the trace amount of oxygen contained in the nitrogen gas, 2CuO + H2 = Cu2O + H2O 2Cu2O + O2 + N2 = 4CuO + N2.
This product can be reacted with concentrated hydrochloric acid, generating white crystalline powder of cuprous chloride.
The industrial manufacturing process of cuprous oxide is to use the metal copper to reduce the copper oxide mineral, or take the copper as the electrode to electrolyze the sodium chloride solution. Cuprous oxide is toxic and can be used as a germicide for agricultural crops; it can be incorporated into the ship bottom paint to prevent the growth of algae and shellfish attached to the bottom of the ship. Cuprous oxide has semiconductor properties and is commonly used to be packed with copper into a cuprous rectifier. Cuprous oxide, acetic acid and ammonia can be formulated into acetate diammonium solution, being a excellent copper ammonia lotion being able to absorb carbon monoxide, used to wash away small amount of the carbon monoxide impurities during the production of hydrogen gas using water gas system in small-scale ammonia plant. Preparation and absorption reaction:
Cu2O + 2HAc + 4NH3 = 2 [Cu (NH3) 2] Ac + H2O
[Cu (NH3)2] Ac + CO + NH3 = [Cu (NH3) 3] Ac • CO
The cuprammonium lotion, after absorbing the carbon monoxide, after heat treatment under reduced pressure, can be recycled for usage. The diamminecopper coordination ion solution formulated by cuprous oxide is easy to react with oxygen, being commonly used as a gas deaerator.
Germicide
Cuprous oxide is a protective germicide, being able to effectively inhibit mycelial growth and cause destruction of its reproductive organs, being able to prevent the spread. It can be used for seed treatment and foliar spray. It can be used in seeds dressing for the prevention of powdery mildew, leaf spot disease, blight, scab and rot disease. It can be applied to the seed soaking of spinach, beet, tomato, pepper, pea, pumpkin, kidney bean and melon as well as to spraying for preventing and curing fruit tree disease. It can also be used for seed dressing, killing slugs and snails.
Toxicity
This product dust, when presented at a air content of being 0.22~14 mg/m3, can cause acute poisoning at 1~2 h after work, manifested as headache, weakness, pharynx and conjunctival redness, nausea, muscle pain, and sometimes vomiting and diarrhea, fatigue and the increase of the body temperature. At one day later, the body temperature can return to normal, but the people can still feel weak and suffer from headache, dizziness, rapid pulse and increased lymphocytes. Chronic poisoning is manifested as: the local skin, hair and conjunctiva of workers exposed to copper compounds sometimes become yellowish green or dark green; the gum of them exhibit dark red or crimson edge.
It is irritant to skin with the dust can stimulate to the eyes and cause corneal ulcers.
For acute poisoning, people should use certain amount of K4 [Fe (CN) 6] solution for gastric lavage or drink milk.
The maximum allowable concentration in air is 0.1 mg/m3.
People can wear masks, dust-proof glasses, wear protective overalls. People need to take shower after work.
Chemical properties
It appears as red or dark red octahedral cubic crystalline powder. It is insoluble in water and alcohol, soluble in hydrochloric acid, ammonium chloride, and ammonia and slightly soluble in nitric acid. It, when dissolved in hydrochloric acid, can produce whitecrystalline cuprous chloride powder. It can be dissolved in concentrated alkali and ferric chloride solution.
Uses
1. It can be used for coating of ship bottom coating, pesticide germicide, and glaze and used for copper oxide rectifier, photocell, electroplating and copper salt production.
2. It can be used in the manufacture of antifouling paint (used to kill low-level marine animals), pesticides, and various copper salts, analytical reagents and red glass. It can also be used in the rectifier plating in the electrical industry. It can also be used as the coloring agent for ceramic and enamel.
3. It can be used as analytical reagents and germicide.
4. It can be used as the reducing agent for determination of nitrogen content in azo compounds, red glaze, electroplating, germicide, red glass, ship bottom paint, plant seeds sterilization and catalyst.
Production method
Dry copper powder, after the removal of impurities, is mixed with copper oxide and sent into the calcination furnace for being heated to 800~900 ℃ to be calcined into cuprous oxide. After taking out, use a magnet to suck the mechanical impurities and then smashed to 325 meshes, making cuprous oxide products. If taking copper sulfate as raw materials, we should first use iron to reduce the copper contained in the copper sulfate out. The subsequent reaction steps are the same as that of the method using copper powder as raw material. Its reaction process is:
Cu + CuO→Cu2O
Glucose reduction method will be copper sulfate solution mixed with glucose and sodium hydroxide solution was added to the reaction, the formation of cuprous oxide, filtered, rinsed, dried crushed system in the cuprous oxide products.
CuSO4 + 2NaOH → Na2SO4 + Cu (OH) 2 ↓
2Cu (OH) 2 + CH2OH (CHOH) 4CHO → Cu2O ↓ + 2H2O + CH20H (CHOH) 4COOH
Electrolytic method: in the iron-lined electrolytic cell with PVC lining, copper plate is used as anode and copper plate as cathode, potassium chromate is used as additive and salt solution as electrolyte, which contains 290 to 310 g/L of sodium chloride, 0.3 to 0.5 g/L of potassium chromate. Electrolysis is carried out under the condition that the temperature is 70-90 °C, Ph is 8 to 12 and the current density is 1500 A/m2, we can produce cuprous oxide with the precipitate being separated, rinsed, filtered and dried to obtain cuprous oxide. Its reaction is:
Cathode reaction: 2H ++ 2e → H2O ↑
Anodic reaction: Cu-2e → Cu +
2Cu ++ 2C1-→ Cu2C12
Cu2C12 + 2NaOH → Cu2O ↓ + H2O + 2NaCl
Hazards & Safety Information
Category : Pesticides
Toxicity grading: highly toxic
Acute Toxicity: Oral-rat LD50 470 mg/kg; celiac-mouse LD50: 380 mg/kg
Flammability and Hazardous properties: Non-combustible with fire producing toxic copper-containing fumes
Storage and transportation characteristics : Treasury should be of low temperature, good ventilation and being drying; store it separately from food raw materials.
Fire extinguishing agent : water, carbon dioxide, dry powder, sand
Professional Standard : TWA 0.1 mg Cu/m3; STEL 0.2 mg Cu/m3
Chemical Properties
Copper(I) oxide, Cu2O, occurs in nature as the red or reddish brown mineral cuprite in a cubic or octahedral crystalline morphology. Synthetic materials variously appear as yellow (somewhat pyrophoric), orange, red, or purple powders, depending on particle size. Copper(I) oxide is stable in dry air, but slowly oxidizes to copper(II) oxide, [1317-38-0], in moist air. It is virtually insoluble in water but dissolves in complexing acids such as HCl and in aqueous ammonia to form copper(I) complexes. In dilute sulfuric and nitric acids, disproportionation to the soluble copper(II) salts and copper powder obtains.
Uses
Red pigment for glass and ceramic glazes, and as a catalystCopper(I) oxide is used in antifouling, marine paint, brazing paste, antifungal treatment for plant seeds. Used as a hydrogen scavenger in copper brass and bronze and high grade lubricants. Finds uses in glass and ceramic pigments, ferrite magnets, catalyst in organic synthesis, dyes and dye intermediates and as catalysts.
Uses
Copper (I) oxide is used as red pigment for glass; in antifouling paints; fungicide.
Uses
Fungicide; antiseptic for fishnets; in antifouling paints for marine use; in photoelectric cells; as red pigment for glass, ceramic glazes; in brazing pastes; in rectifiers; as catalyst.
Definition
An insoluble red powder prepared by the heating of copper with copper(II) oxide or the reduction of an alkaline solution of copper(II) sulfate. Copper(I) oxide is easily reduced by hydrogen when heated; it is oxidized to copper(II) oxide when heated in air. It is used in the glass industry. Copper( I) oxide undergoes disproportionation in acid solutions, producing copper(II) ions and copper. The oxide will dissolve in concentrated hydrochloric acid due to the formation of the complex ion [CuCl2]-. Copper(I) oxide is a covalent solid.
Preparation
Copper(I) oxide decomposes above 1800°C but can be prepared by heating copper metal in air above 1030°C. To prevent further oxidation, the material must be cooled in an inert atmosphere. Lower temperatures may be used to produce the oxide if the material is blended with a reducing agent such as carbon and heated to 750°C in an inert atmosphere. The product must be stabilized with isophthalic acid or pine oil (Ayers, 1953). A particularly stable copper(I) oxide can be prepared by blending copper powder and cupric oxide in stoichiometric amounts and heating to 800-900°C. Lower temperatures can be used if ammonia or certain ammonium salts are added to the blend (Day, 1969; Drapeau and Johnson, 1956; Drapeau and Johnson, 1959).
Definition
For the native ore see cuprite.
General Description
Copper(I) oxide is a weak inorganic base that can be used to activate halides for nucleophilic substitution reaction. It is also used in decarboxylation and cyclo condensation reactions.
Hazard
Toxic by ingestion.
Safety Profile
Moderately toxic by ingestion. Experimental reproductive effects. A fungicide. Violent, potentially explosive reaction with concentrated peroxyformic acid. Violent reaction when heated with aluminum. See also COPPER COMPOUNDS.
Cuprous oxide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| WUXI HONOR SHINE CHEMICAL CO.,LTD | +86-0510-83593312 +8619805123561 | sales@honorshinechem.com | China | 60 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3878 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5873 | 58 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | aroma@qdtrustagri.com | China | 299 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 645 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
Related articles
- Cuprous Oxide: A Comprehensive Overview for the Chemistry Professional
- Cuprous Oxide is a versatile and vital chemical compound with extensive applications in both industrial and agricultural setti....
- Jun 25,2024
- Cuprous oxide nanoparticles
- CuO engineered nanoparticles (ENPs) are used in industrial applications as a catalyst for carbon monoxide oxidation and a comp....
- Mar 17,2023
- Uses and properties of Cuprous oxide
- Cuprous oxide is a narrow bandgap (1.8–2.2 eV) p-type semiconductor that is abundant and cheap. Because of this, it can also a....
- Jun 15,2022
View Lastest Price from Cuprous oxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | Cuprous oxide
1317-39-1
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-13 | Cuprous oxide
1317-39-1
|
US $6.00-3.50 / kg | 1kg | 99.99% | 10 tons | Shandong Deshang Chemical Co., Ltd. | |
 |
2024-09-04 | Cuprous oxide
1317-39-1
|
US $0.00-0.00 / KG | 1KG | 99.0% | 10000KG | Shaanxi Dideu Medichem Co. Ltd |
-

- Cuprous oxide
1317-39-1
- US $5.00-2.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Cuprous oxide
1317-39-1
- US $6.00-3.50 / kg
- 99.99%
- Shandong Deshang Chemical Co., Ltd.
-

- Cuprous oxide
1317-39-1
- US $0.00-0.00 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
1317-39-1(Cuprous oxide)Related Search:
1of4