tert-ブチルメチルエーテル 化学特性,用途語,生産方法
外観
無色澄明の液体
溶解性
水に不溶。殆どの有機溶媒に可溶。エタノール及びアセトンに極めて溶けやすく、水にやや溶けやすい。水 5.126 vol% (25℃)。
用途
液体クロマトグラフ分析による中性脂肪、遊離脂肪酸の分析における溶離液及び溶離液調製用。
用途
食品及び水中等の農薬及びPCB定量における溶媒。
用途
低沸点溶剤。有機合成原料。
用途
分光分析用。
用途
ガソリンのオクタン価向上剤
用途
ガスクロマトグラフ分析による水中のジクロロ酢酸等の定量における抽出溶媒。
用途
残留農薬・PCB 試験用溶媒。
使用上の注意
不活性ガス封入
化学的特性
tert-Butyl methyl ether, also known as MTBE or Methyl tert-butyl ether, is a clear, colorless liquid with a low viscosity that is combustible and has a distinct, turpentine-like odor. It is miscible with organic solvents, but only slightly soluble in water. Methyl tert-butyl ether is very stable under alkaline, neutral, and weakly acidic conditions. In the presence of strong acids, it is cleaved to methanol and isobutene. Depending on reaction conditions the latter can form isobutene oligomers. MTBE does not undergo autoxidation and, in contrast to other ethers, it does not form peroxides with atmospheric oxygen. It improves the antiknock properties when added to motor gasoline.
来歴
tert-Butyl methyl ether was first synthesized (by the classical Williamson ether synthesis) and characterized in 1904. Extensive studies in the United States during World War II demonstrated the outstanding qualities of MTBE as a high-octane fuel component. It was first commercially produced in Italy in 1973 for use as an octane enhancer in gasoline. U.S. production of MTBE started in 1979 after Atlantic Richfield Co. (ARCO) was granted a waiver by the U.S. Environmental Protection Agency (EPA) that allowed MTBE to be blended up to 7 vol % in U.S. unleaded gasoline. The use of other aliphatic ethers was allowed when the U.S. EPA issued its “substantially similar” definition for unleaded gasoline specifications in 1981. Under this definition, any aliphatic ether or ether mixture could be blended in unleaded gasoline as long as the total oxygen contribution from the ethers does not exceed 2.0% oxygen by weight in the gasoline. This allowed MTBE to be blended up to approximately 11 vol % in gasoline.
使用
Methyl tert-butyl ether (MTBE) was primarily used as a gasoline additive in unleaded gasoline in the United States prior to 2005, in the manufacture of isobutene, and as a chromatographic eluent especially in high pressure liquid chromatography. It is also a pharmaceutical agent and can be injected into the gallbladder to dissolve gallstones (ATSDR, 1996). tert-Butyl methyl ether is also used in the petrochemical industry. By reversal of its formation reaction, MTBE can be cracked to isobutene and methanol on acidic catalysts at higher temperature than MTBE synthesis.
製造方法
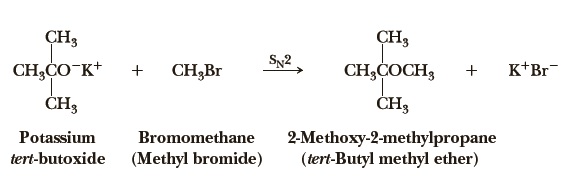
tert-butyl methyl ether can be prepared by the reaction of potassium tert-butoxide and bromomethane. Methyl tert-butyl ether also can be obtained by the acid-catalyzed addition of methanol to isobutene. Suitable catalysts are solid acids such as bentonites, zeolites and – commonly used in industrial world scale MTBE-production units – macroporous acidic ion-exchange resins. The reaction is weakly exothermic with a heat of reaction of -37.7 kJ/mol.
定義
ChEBI: Methyl tert-butyl ether is an ether having methyl and tert-butyl as the two alkyl components. It has a role as a non-polar solvent, a fuel additive and a metabolite.
主な応用
tert-Butyl methyl ether (MTBE) is a gasoline additive. MTBE undergoes oxidative degradation in the presence of propane-oxidizing bacterial strains. The kinetic studies of heat-assisted persulfate oxidation of MTBE under various parameters suggests that the reaction follows the pseudo-first-order kinetics. MTBE can be synthesized by acid catalyzed reaction between methanol and isobutene. It is an effective alternative to lead containing additives for enhancing the octane rating of gasoline. A study suggests that the addition of MTBE increases the number of active sites during polymerization of propene by stopped-flow method.
tert-Butyl methyl ether may be used to synthesize fatty acid methyl esters (FAMEs) and glycerol tert-butyl ether via transesterification with canola oil under supercritical conditions.
一般的な説明
Methyl tert-butyl ether appears as a colorless liquid with a distinctive anesthetic-like odor. Vapors are heavier than air and narcotic. Boiling point 131°F. Flash point 18°F. Less dense than water and miscible in water. Used as a octane booster in gasoline.
空気と水の反応
Highly flammable. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979. p.151-154, 164]. A mixture of liquid air and diethyl ether exploded spontaneously [MCA Case History 616. 1960].
反応プロフィール
Ethers, such as tert-Butyl methyl ether, can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.
危険性
Slightly toxic by ingestion and inhalation.
Flammable when exposed to heat or flame. Upper
respiratory tract irritant and kidney damage. Questionable carcinogen.
健康ハザード
INHALATION: May cause dizziness or suffocation. Contact may irritate or burn eyes or skin. May be harmful if swallowed.
工業用途
Methyl tert-butyl ether (MTBE) is used as an octane enhancer in gasoline. EPA
regulations allow up to 2.7 wt.% oxygen in gasoline which allows 15 vol.%
MTBE in gasoline. Other alkyl ethers can also be blended into gasoline up to the
2.7 wt% oxygen requirement. The stability of MTBE to oxidation and peroxide
formation gives this unsymmetrical ether an advantage over other ethers in various
extraction and reaction solvent applications.
安全性プロファイル
Poison by intravenous
route. Slightly toxic by ingestion and
inhalation. Flammable when exposed to heat
or flame. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also ETHERS.
職業ばく露
Used as an organic solvent; as an
octane booster in unleaded gasolines; in making other chemicals; and in medicine to dissolve gall stones
発がん性
EPA has not classified methyl tert-butyl ether with respect to potential carcinogenicity. There is evidence for the carcinogenicity of MTBE in animals. MTBE causes leukemias/lymphomas in female rats, renal tubular tumors and Leydig cell tumors in male rats, and hepatocellular tumors in mice. Positive animal carcinogenicity data and some further concordance in tumor sites for formaldehyde and TBA, metabolites of MTBE, provide support for this conclusion. However, uncertainties remain about the nature and extent of risk at very low doses, and about the particular tumor sites that are most relevant to humans.
環境運命予測
tert-Butyl Methyl Ether can be released during manufacturing or blending with gasoline; during the storage, distribution, and transfer of MTBE-blended gasoline; and from spills or leaks or fugitive emissions at automotive service stations (U.S. EPA, 1994a). Vapor emissions from MTBE-blended gasoline may also contribute to atmospheric levels (U.S. EPA, 1988). It is not expected to persist in the atmosphere because it undergoes destruction from reactions with hydroxyl radicals. A total atmospheric lifetime for MTBE of approximately 3 and 6.1 days has been reported in polluted urban air and in nonpolluted rural air, respectively (U.S. EPA, 1993a). Based upon its vapor pressure and Henry s law constant, MTBE is highly volatile and would be expected to evaporate rapidly from soil surfaces or water. It may be fairly persistent when introduced into subsurface soils or to groundwater since volatilization to the atmosphere is reduced or eliminated. It does not readily degrade in surface waters due to hydrolysis or other abiotic processes. It is also resistant to biodegradation (U.S. EPA, 1993a). It is usually removed from surface waters very rapidly because of its high volatility. If released as part of a gasoline mixture from leaking underground storage tanks, its relatively high water solubility combined with little tendency to sorb to soil particles encourages migration to local groundwater supplies (U.S. EPA, 1993a).
輸送方法
UN2398 Methyl tert-butyl ether, Hazard Class: 3;
Labels: 3-Flammable liquid.
純化方法
Purify as for n-butyl methyl ether. [Beilstein 1 IV 1615.]
不和合性
May form explosive mixture with air.
May be able to form unstable peroxides. Much less likely
to form peroxides than other ethers. Incompatible with
strong acids. Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. May accumulate static
electrical charges, and cause ignition of its vapors.
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be observed.
tert-ブチルメチルエーテル 上流と下流の製品情報
原材料
準備製品
2-アミノチオフェン-3-カルボン酸メチル
(1R,2R)-(-)-1,2-シクロヘキサンジアミン
1-[(TERT-ブトキシカルボニル)アミノ]シクロプロパンカルボン酸
(1R,1β,4β)-2-アザビシクロ[2.2.1]ヘプタ-5-エン-3-オン
2-(ブロモメチル)アクリル酸エチル
5-メトキシ-1H-ピロロ[3,2-B]ピリジン-2-カルボン酸
クロロぎ酸2,2,2-トリクロロエチル
BIS(4-NITROBENZYL) PHOSPHOROCHLORIDATE
トリス(ジベンジリデンアセトン)(クロロホルム)ジパラジウム(0)
3-N-BOC-アミノアゼチジン
4-(クロロメチル)スチレン
N,N-ジエチルアセトアセタミド
ナトリウムトリアセトキシボロヒドリド
ブリンゾラミド
1H-インドール-5-アミン/塩酸,(1:x)
4-アミノテトラヒドロピラン塩酸塩
N-メチル-p-アニシジン
3-アミノ-4-ピリジンカルボン酸エチル
2-クロロマロンアルデヒド
アゼチジン
4-PYRIDIN-2-YLISOXAZOL-5-AMINE
2-(2-ニトロフェニル)プロペナール
3-アミノ-4-ヒドロキシメチルピリジン
テトラヘプチルアンモニウムブロミド
テトラキス(トリフェニルホスフィン)パラジウム(0)
5-METHOXY-1H-PYRROLO[2,3-C]PYRIDINE-2-CARBALDEHYDE
1-アミノシクロプロパンカルボン酸
5-メトキシ-1H-ピロロ[2,3-C]ピリジン-2-カルボン酸
トリクロロオクタデシルシラン
6-ニトロインドール
ω-ホスホノ-L-アルギニン
プロパン二酸1-(1,1-ジメチルエチル)3-メチル
ヘキサフルオロアセチルアセトン
6-アミノインドール
アゼチジン塩酸塩
1-メトキシ-3-(ブロモメチル)ベンゼン
4,6-ジメチルピリミジン-5-カルボン酸
酢酸2-メトキシエチル
3-アミノピリジン-4-カルボキシアルデヒド
PHOSPHO-L-ARGININE SODIUM