シアル酸 化学特性,用途語,生産方法
外観
白色~わずかにうすい褐色, 結晶性粉末~粉末
定義
本品は、次の化学式で表される有機化合物である。
溶解性
水に溶けやすく、エタノール及びメタノールに溶けにくく、アセトンにほとんど溶けない。
解説
シアル酸は,ノイラミン酸群の総称で,シアリン酸ともいう。ウシの顎下腺ムチンから最初に分離された (1936) 。酸性ムコ多糖類の一成分で,マンノースアミンとピルビン酸の縮合物である。ノイラミン酸 (分子式 C9H17NO8 ) のほか,o- ,p- ,e- ,b- シアル酸などが知られている。血液型物質などの糖蛋白質の糖鎖の非還元末端に存在していて,ノイラミニダーゼ (シアリダーゼ) 処理によって容易にはずすことができる。インフルエンザウイルスによる赤血球凝集反応を阻害することで知られていたが,これはウイルス表面のノイラミニダーゼスパイクに作用するためである。
単糖の一種で、1分子中にカルボキシ基(カルボキシル基)、ケト基(カルボニル基)、アセトアミド基をもつ複雑な構造をしている。代表例はN-アセチルノイラミン酸で、これはピルビン酸とN-アセチルマンノサミンのアルドール縮合体と考えられる。シアル酸の性質のうちでとくに重要なことは、カルボキシ基の存在である。シアル酸は糖タンパク質、糖脂質(ガングリオシド)の非還元末端に幅広く分布し、これらに酸性の性質を与えている。また細胞表面の陰電荷のかなりの部分はシアル酸に起因している。
分子中にカルボキシル基・カルボニル基・アセトアミド基をもつ、複雑な構造の単糖。糖たんぱく質や糖脂質などの糖鎖の末端に存在し、多様な生理現象に関与している。インフルエンザウイルスは、シアル酸を末端にもつ糖鎖を受容体として宿主細胞に吸着し、細胞内に取り込まれ増殖した後、ノイラミニダーゼという酵素によってシアル酸から切り離され、宿主細胞外に放出されて他の細胞に感染する。
解説
シアリン酸ともいう.ノイラミン酸誘導体の総称.ムコ多糖,糖タンパク質,糖脂質,人乳のオリゴ糖などの構成成分として広く動物界に分布している.
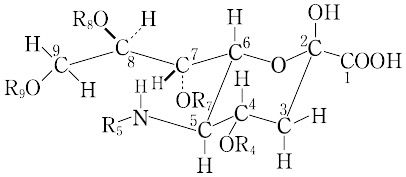
"ノイラミン酸はα結合で,糖鎖の非還元末端に結合しているが,図の R4~R9 がアセチル化されるほかに,R5 がグリコリル化,R8 がメチル化および硫酸化,R9 がラクチル化およびリン酸化された20種類以上の誘導体が知られている.これらは弱アルカリで,ヘキソサミンとピルビン酸に分解し,前処理なしに直接エールリヒ試薬により紫色を示す.不飽和の2-デオキシ-2,3-デヒドロ-N-アセチルノイラミン酸も知られているが,N-アセチルノイラミン酸がもっとも代表的なものであり,生化学的には可逆的酵素反応によりN-アセチル-D-マンノサミンとホスホエノールピルビン酸から合成あるいは分解される.シアル酸含有複合糖質では,シアリダーゼによるシアル酸の除去により,はじめて引き続く糖鎖の分解が可能になる.シアル酸は糖タンパク質に高い粘度を付与し,細胞表層の陰性荷電に大きく寄与し,細胞膜の営む種々の機能に関与している.
森北出版「化学辞典(第2版)
化粧品の成分用途
皮膚コンディショニング剤、皮膚保護剤
効能
筋疾患治療薬
説明
N-acetylneuraminic acid is an N-acyl derivative of neuraminic or acid amino sugar derivative, derived from N-acetylmannosamine and pyruvic acid. It is an important constituent of glycoproteins and glycolipids. N-acetylneuraminic acid occurs in many polysaccharides, glycoproteins, and glycolipids in animals and bacteria.
物理的性質
The numbering of the sialic acid structure begins at the carboxylate carbon and continues around the chain. The configuration which places the carboxylate in the axial position is the alpha-anomer.
The alpha-anomer is the form that is found when sialic acid is bound to glycans, however, in solution it is mainly (over 90 %) in the beta-anomeric form. A bacterial enzyme with sialic acid mutarotase activity, NanM, has been discovered which is able to rapidly equilibrate solutions of sialic acid to the resting equilibrium position of around 90 % beta 10 % alpha.
生合成
In bacterial systems, sialic acids are biosynthesized by an aldolase enzyme. The enzyme uses a mannose derivative as a substrate, inserting three carbons from pyruvate into the resulting sialic acid structure. These enzymes can be used for chemoenzymatic synthesis of sialic acid derivatives.
生物学の機能
Sialic acid-rich glycoproteins (sialoglycoproteins) bind selectin in humans and other organisms. Metastatic cancer cells often express a high density of sialic acid-rich glycoproteins. This overexpression of sialic acid on surfaces creates a negative charge on cell membranes. This creates repulsion between cells (cell opposition) and helps these late-stage cancer cells enter the blood stream.
Sialic acid also plays an important role in human influenza infections.
Many bacteria also use sialic acid in their biology, although this is usually limited to bacteria that live in association with higher animals (deuterostomes). Many of these incorporate sialic acid into cell surface features like their lipopolysaccharide and capsule, which helps them evade the innate immune response of the host.[6] Other bacteria simply use sialic acid as a good nutrient source, as it contains both carbon and nitrogen and can be converted to fructose-6- phosphate, which can then enter central metabolism.
Sialic acid-rich oligo saccharides on the glyco conjugates ( glyco lipids, glyco proteins, proteoglycans) found on surface membranes help keep water at the surface of cells . The sialic acid - rich regions contribute to creating a negative charge on the cells' surfaces. Since water is a polar molecule with partial positive charges on both hydrogen atoms, it is attracted to cell surfaces and membranes. This also contributes to cellular fluid uptake.
Sialic acid can "hide" mannose antigens on the surface of host cells or bacteria from mannose - binding lectin . This prevents activation of complement.
Sialic acid in the form of poly sialic acid is an unusual posttranslational modification that occurs on the neural cell adhesion molecules (NCAMs). In the synapse, the strong negative charge of the polysialic acid prevents NCAM cross-linking of cells.
シアル酸 上流と下流の製品情報
原材料
準備製品