アクリロニトリル 化学特性,用途語,生産方法
外観
無色~わずかにうすい黄色, 澄明の液体
物化的性質
アクリロニトリル(Acrylonitrile)とは、ANと略される有機化合物で甘いにおいのする無色透明(光に反応し徐々に淡い黄色へ変わる)の液体で、ほとんどの有機溶剤に溶ける(特にエタノールやアセトンには顕著)ことで知られています。反応性が高く、付加反応によりシアノエチル基-CH2CH2CNを導入するシアノエチル化剤となる。重合や共重合しやすく、重合体のポリアクリロニトリルは合成繊維として、ブタジエンとの共重合体は合成ゴム(NBR)として、ブタジエン・スチレンとの共重合体はABS樹脂として用いられる。そのほか炭素繊維、塗料、有機合成原料となる。可燃性であるうえに、空気に3~17%混和すると爆発するので、換気のよい所で保存し、取扱いに注意する。
反応
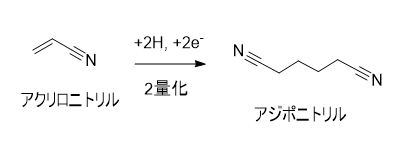
主にポリアクリロニトリルなどの重合体を生成するためのモノマーとして反応する。重合以外の反応性として、アクリロニトリルの構造的特徴から、マイケル付加(1,4付加)を受けやすい。そのためシアノエチル化剤として有用です。また、二量化させることでが得られ、これはの原料として利用されています。
溶解性
水に可溶 (9.3g/100g水, 20℃), ほとんどの有機溶剤に易溶。エタノール及びアセトンに極めて溶けやすく、水にやや溶けやすい。
解説
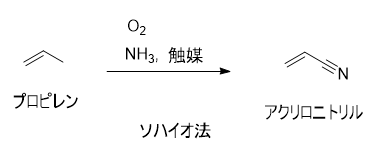
アクリロニトリルは、分子式CH2=CHCN、分子量53.06、融点-83〜-84℃、沸点約78℃、中間体として化学工業界でも重要な物質ですが、毒性や引火性(引火点-1℃)でもあることから消防法の第4類第1石油類、毒物及び劇物取締法により劇物に含まれ、安全衛生法では有害物質表示対象物質などに指定されています。工業的に,初期にはアセチレンと青酸の反応で合成されたが,ソハイオ法の出現以来,すべてプロペンのアンモ酸化反応によって合成されるようになった.C3H6+NH3+3/2O2--->CH2=CHCN+3H2O. "触媒としては,まずモリブデン酸ビスマス,またはリンモリブデン酸ビスマスが用いられたが,その後,多くの改良触媒が開発されている.特異臭をもつ無色の液体.融点-83 ℃,沸点77.3 ℃.d204 0.8060.n20D 1.3911.多くの有機溶剤に易溶.蒸気は有毒性で,空気中20 ppm 以上は危険である.アクリル系合成繊維の単量体として工業的に大量に使用され,またブタジエンと共重合して合成ゴムNBR,ブタジエン,スチレンと共重合してABS樹脂が製造される.このほか,各種の高分子原料,有機合成原料として使用される.[CAS 107-13-1]
森北出版「化学辞典(第2版)
用途
合成繊維?アクリロニトリル-ブタジエン-スチレン(ABS)樹脂?アクリロニトリル-スチレン(AS)樹脂原料,合成ゴム (ニトリルゴム)樹脂原料、塗料?繊維樹脂加工?化粧品原料?合成糊料合成原料、アクリルアミド(紙力増強剤,凝集剤)重合原料、NITE初期リスク評価書;合成繊維?合成ゴム?プラスチック原料、アクリルアミド?アジポニトリル原料、SRI:CHEMICAL ECONOMICS HANDBOOK;合成繊維?合成ゴム?プラスチック原料 (化学工業日報社)
製法
工業的にはSOHIO Process (ソハイオ法)と呼ばれる、を金属酸化物触媒存在下に、と酸素を作用させ生産します。、シアン化水素が副生され、これらも工業製品として利用されています。
化学的特性
Acrylonitrile is a colorless, flammable liquid. Its vapors may explode when exposed to an
open flame. Acrylonitrile does not occur naturally. It is produced in very large amounts
by several chemical industries in the United States and its requirement and demand has
increased in recent years. The largest users of acrylonitrile are chemical industries that make
acrylic and modacrylic fi bers, high impact acrylonitrile-butadiene-styrene (ABS) plastics.
Acrylonitrile is also used in business machines, luggage, and construction material, in the
manufacturing of styrene-acrylonitrile (SAN) plastics for automotive and household goods,
and in packaging material. Adiponitrile is used to make nylon, dyes, drugs, and pesticides.
使用
Acrylonitrile is used in the production of acrylic fibers, resins, and surface coating; as an intermediate in the production of pharmaceuticals and dyes; as a polymer modifier; and as a fumigant. It may occur in fire-effluent gases because of pyrolyses of polyacrylonitrile materials. Acrylonitrile was found to be released from the acrylonitrile–styrene copolymer and acrylonitrile–styrene–butadiene copolymer bottles when these bottles were filled with food-simulating solvents such as water, 4% acetic acid, 20% ethanol, and heptane and stored for 10 days to 5 months (Nakazawa et al. 1984). The release was greater with increasing temperature and was attributable to the residual acrylonitrile monomer in the polymeric materials.
定義
ChEBI: Acrylonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by an ethenyl group. It is very toxic and irritant but is also a sensitizer. It caused both irritant and allergic contact dermatitis in a production manufacture.
空気と水の反応
Highly flammable. Soluble in water.
反応プロフィール
ACRYLONITRILE produces poisonous hydrogen cyanide gas on contact with strong acids or when heated to decomposition. Reacts violently with strong oxidizing agents (dibenzoyl peroxide, di-tert-butylperoxide, bromine) [Sax, 9th ed., p. 61]. Rapidly ignites in air and forms explosive mixtures with air. Polymerizes violently in the presence of strong bases or acids. Underwent a runaway reaction culminating in an explosion on contact with a small amount of bromine or solid silver nitrate [Bretherick, 5th ed., 1995, p. 404].
使用用途
アクリロニトリルは、強酸や強塩基などと活発に重合し、活性な二重結合をもち環状化合物を形成することから、医薬品はじめ染料や塗料などのほか、軽量かつ強度を求められる航空機などでお馴染みのの原料としても使われています。
アクリロニトリルの重合体である(PAN)は、アクリル繊維の主成分としてセーターや靴下といったニット製品に使用されています。
また、プラスチック素材としてABS樹脂、AS樹脂などの合成樹脂の原料として使用されています。タイヤ・パッキン・コンベアベルトなどへの合成ゴムの主要原料としても使われています。
化学性质
色の変質しやすい液体で、甘い臭気がする。高純度品は室温で重合する。
接触アレルゲン
Acrylonitrile is a raw material used extensively in industry,
mainly for acrylic and modacrylic fibers, acrylonitrile-butadiene-styrene
and styrene-acrylonitrile resins,
adiponitrile used in nylon’s synthesis, for nitrile rubber,
and plastics. It is also used as an insecticide. This very
toxic and irritant substance is also a sensitizer and caused
both irritant and allergic contact dermatitis in a production
manufacturer.
職業ばく露
Acrylonitrile is used in the manufacture of synthetic fibers, polymers, acrylostyrene plastics, acrylonitrile butadiene styrene plastics, nitrile rubbers, chemicals, and adhesives. It is also used as a pesticide. In the past, this chemical was used as a room fumigant and pediculicide (an agent used to destroy lice).
発がん性
Acrylonitrile is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
貯蔵
Work with acrylonitrile
should be conducted in a fume hood to prevent exposure by inhalation, and splash
goggles and impermeable gloves should be worn at all times to prevent eye and skin
contact. Acrylonitrile should be used only in areas free of ignition sources.
Containers of acrylonitrile should be stored in secondary containers in the dark in
areas separate from oxidizers and bases.
輸送方法
UN1093 Acrylonitrile, stabilized, Hazard Class 3; Labels: 3 Flammable liquids, 6.1-Poisonous materials
合成方法
リン、モリブデン酸、スズ、アンチモンの塩(ほかにも鉄、アンチモン系など数多くの改良触媒が使用されている)を主体とした触媒をシリカに担持させたものを用いて、500℃以下0.3 MPa以下で数秒間反応させて得る。
純化方法
Wash acrylonitrile with dilute H2SO4 or dilute H3PO4, then with dilute Na2CO3 and water. Dry it with Na2SO4, CaCl2 or (better) by shaking with molecular sieves. Fractionally distil it under N2. It can be stabilised by adding 10ppm tert-butyl catechol. Immediately before use, the stabilizer can be removed by passage through a column of activated alumina (or by washing with 1% NaOH solution if traces of water are permissible in the final material), followed by distillation. Alternatively, shake it with 10% (w/v) NaOH to extract inhibitor, and then wash it in turn with 10% H2SO4, 20% Na2CO3 and distilled water. Dry for 24hours over CaCl2 and fractionally distil under N2 taking fraction boiling at 75.0-75.5oC (at 734mm). Store it with 10ppm tert-butyl catechol. Acrylonitrile is distilled off when required. [Burton et al. J Chem Soc, Faraday Trans 1 75 1050 1979, Beilstein 2 IV 1473.]
不和合性
Acrylonitrile is reactive with, and must be kept away from, strong oxidizers, especially bromine. Use extreme care to keep Acrylonitrile away from strong bases, strong acids, copper, copper alloys, ammonia and amines. Contact with these chemicals can cause a chemical reaction resulting in a fire or explosion. Chemical compatibility should also be determined before Acrylonitrile comes in contact with any other chemical.
廃棄物の処理
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with provision for nitrogen oxides removal from effluent gases by scrubbers or afterburners. A chemical disposal method has also been suggested involving treatment with alcoholic NaOH; the alcohol is evaporatedand calcium hypochlorite added; after 24 hours the product is flushed to the sewer with large volumes of water. Recovery of acrylonitrile from acrylonitrile process effluents is an alternative to disposal.
アクリロニトリル 上流と下流の製品情報
原材料
準備製品
グリコール酸でん粉ナトリウム
1-(2-シアノエチル)ピペラジン
Leather lustering agent
フルスルチアミン
シベンゾリン
N,N-ジエチルエチレンジアミン
1-(3-アミノプロピル)イミダゾール
Chlorpyrifos,E.C.
N-(ETHOXYCARBONYL)-N-(ETHOXYCARBONYKLETHYL)GLYCINE ETHYL ESTER
3-(1H-ピロール-1-イル)プロパンニトリル
N-(ヒドロキシメチル)アクリルアミド
2-ピペリドン-3-カルボン酸エチル
3-ジメチルアミノプロピオニトリル
3,3'-チオジプロピオン酸ジドデシル
3-クロロプロピオニトリル
Polyacrylamide dry powder,cationic
2-chloro-2-trichloroethyl-3,3-dimethyl cyclobutenone
modified acrylic resin emulsion J
rubber latex BA
acrylic resin emulsion FX-1
Nitrile rubber NBR
crosslinking aent DTF-3
3,3'-(フェニルイミノ)ビスプロパンニトリル
coupling agent NBC-1
rac-(R*)-2,3-ジブロモプロピオノニトリル
brubber latex 202BA
2-(2-シアノエチル)マロン酸 ジエチル
フルジオキソニル
1,4-ビス(3-アミノプロピル)ピペラジン
Methyl 1-Methyl-2-oxopyrrolidine-3-carboxylate
N-(2-シアノエチル)グリシン
2,2'-チオジグリコール酸
Binder for coating printing
2,3-ジクロロプロパンニトリル
Polyacrylamide dry powder,non-ionic
3 - ジエチルアミノプロピルアミン
interpenetrating network ion exchange resin
TXG retention aid
2-ブロモ-2-(ブロモメチル)グルタロニトリル
N,N-ジベンジルヒドロキシルアミン