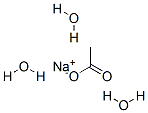
아세트산나트륨, 트리수화물
|
|
아세트산나트륨, 트리수화물 속성
- 녹는점
- 58 °C
- 끓는 점
- >400°C
- 밀도
- 1,45 g/cm3
- 인화점
- >250°C
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 3 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- 냄새
- 가벼운 식초 냄새
- pH 범위
- 8.5 - 10 at 408 g/l at 25 °C
- 수소이온지수(pH)
- 7.5-9.0 (25℃, 50mg/mL in H2O)
- 산도 계수 (pKa)
- 4.76 (acetic acid)(at 25℃)
- 수용성
- 762g/L(20℃)
- 최대 파장(λmax)
- λ: 260 nm Amax: ≤0.01
λ: 280 nm Amax: ≤0.01
- Merck
- 14,8571
- BRN
- 3732037
- 안정성
- 안정적인. 강한 산화제, 할로겐과 호환되지 않습니다.
- InChIKey
- AYRVGWHSXIMRAB-UHFFFAOYSA-M
- LogP
- -0.285 (est)
- CAS 데이터베이스
- 6131-90-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 24/25-36-26 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | AJ4580000 | ||
| 자연 발화 온도 | 607 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29152200 | ||
| 독성 | LD50 orally in Rabbit: 3530 mg/kg LD50 dermal Rabbit > 10000 mg/kg |
아세트산나트륨, 트리수화물 C화학적 특성, 용도, 생산
물성
화학식 CH3COONa· 분자량 82.04, 녹는점 324℃, 비중 1.528이다. 아세트산을 수산화나트륨 또는 탄산나트륨으로 중화하고, 증발·농축하여 냉각시키면 3수화염 CH3CO2Na·3H2O가 생긴다. 이 3수화염의 결정을 120∼250℃로 가열하면 무수염(無水 鹽)이 된다.용도
약한 산과 강한 염기가 만드는 염이므로 수용액은 약한 알칼리성을 보인다. 완충용액의 조제, 염료의 매염제(媒染劑) 로 사용되며, 융해열이 크기 때문에 난방기구의 보온재로도 사용된다.화학적 성질
Sodium acetate trihydrate is the trihydrate sodium salt oaf acetic acid. It is a colorless, odorless crystals that can be weathered in the air. Soluble in water and ether, pH of 0.1M aqueous solution is 8.9. moderately soluble in ethanol, 5.3 g/100mL. Anhydrous Sodium acetate occurs as colorless, transparent crystals or a granular crystalline powder with a slight acetic acid odor.용도
Sodium acetate trihydrate is used in various applications. It is used as a buffer of a pH range in between to 4.0 to 6.0. It is also used in the purification and precipitation of nucleic acids and in protein crystallization. It is used as a mordant dyeing, as a pickling agent in chrome tanning, as a neutralizer in waste streams containing sulfuric acid, in the textile industry and as a photoresist while using aniline dyes. It plays an important role to retard vulcanization of chloroprene in synthetic rubber production. It is a precursor to prepare ester from alkyl halide. Other applications are in photography, as an additive to food, in purification of glucose, in preservation of meat, in tanning, and as a dehydrating agent. In analytical chemistry it is used to prepare buffer solution.제조 방법
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate.NaOH + CH3COOH → CH3COONa + H2O
Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O
일반 설명
Sodium acetate trihydrate can be obtained from the crystallization of sodium acetate in water. On heating at 75°C, it melts, while on heating above 120°C it loses water of crystallization and gets converted into anhydrous form. The hyperfine proton and 13C splittings have been evaluated by recording ESR spectra of its irradiated single crystals. ESR studies suggested the existence of trapped methyl radicals. Its crystals are monoclinic and its crystal structure has been investigated by photographic methods. Unit cell parameters were evaluated.Pharmaceutical Applications
Sodium acetate is used as part of a buffer system when combined with acetic acid in various intramuscular, intravenous, topical, ophthalmic, nasal, oral, otic, and subcutaneous formulations. It may be used to reduce the bitterness of oral pharmaceuticals. It can be used to enhance the antimicrobial properties of formulations; it has been shown to inhibit the growth of S. aureus and E. coli, but not C. albicans in protein hydrolysate solutions. It is widely used in the food industry as a preservative. Sodium acetate has also been used therapeutically for the treatment of metabolic acidosis in premature infants, and in hemodialysis solutions.Safety
Sodium acetate is widely used in cosmetics, foods, and pharmaceutical formulations, and is generally regarded as a nontoxic and nonirritant material.A short-term feeding study in chickens with a diet supplemented with 5.44% sodium acetate showed reduced growth rates that were attributed to the sodium content.(9) Sodium acetate is poisonous if injected intravenously, is moderately toxic by ingestion, and is an irritant to the skin and eyes.
LD50 (rat, oral): 3.53 g/kg
LD50 (mouse, IV): 0.38 g/kg
LD50 (mouse, SC): 8.0 g/kg
저장
Sodium acetate should be stored in airtight containers.비 호환성
Sodium acetate reacts with acidic and basic components. It will react violently with fluorine, potassium nitrate, and diketene.Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections, nasal, otic, ophthalmic, and oral preparations).참고 문헌
[1] http://www.europlat.org[2] http://www.rxlist.com/sodium-acetate-drug.htm
[3] http://cosmetics.specialchem.com
[4] http://cecri.csircentral.net/2710/1/036-96.pdf
아세트산나트륨, 트리수화물 준비 용품 및 원자재
원자재
준비 용품
Gardenia blue pigment
C.I. 색소 황색 83
5-Ethyl-2-thiophenecarboxaldehyde
BISPYRAZOLONE
인산납 디베이직
6-HYDROXY-7-METHOXYCOUMARIN
Pigment Red 32
Pigment Red 13
Pigment Yellow 65
o-Anisic acid
에텐일 옥타데칸산
3-(2-푸릴)이속사졸-5-아민
Pigment Red 175
RHODINYL ACETATE
유연제IS
베타-페닐에틸 아세트산
나트륨 디아세트산
8-메톡시사이마린
초산 토코페놀
초산 게라닐
3-Amino-5-phenylpyrazole
2,6-디티오퓨린
8-(4-아닐리노-5-설포-1-나프틸아조)-1-나프톨-3,6-디술폰 산 트리나트륨 염
glyceryl ethercarboxylic acid salt
3,3'-디하이드록시벤지딘
6-메톡시사이마린
Cefotaxime
PALMITIC ACID VINYL ESTER
2-Acetyl pyrrole
1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
2-아미노-4-(4-클로로-페닐)-티오펜-3-카르복실산에틸에스테르
5-METHYL-4-ISOXAZOLECARBONYL CHLORIDE
4-Hydroxy-3-methoxystyrene
C. I. Pigment Red 258
7-isopropyl-4-methyloxepan-2-one
5-[(1,3-DIOXO-1,3-DIHYDRO-2H-ISOINDOL-2-YL)METHYL]-2-FURALDEHYDE
6-AMINO-5-NITROSO-3-METHYLURACIL
Cephalothin sodium
Coumarin 30
ISOXANTHOPTERIN
아세트산나트륨, 트리수화물 공급 업체
글로벌( 702)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Jiangsu Boquan Biotechnology Co., Ltd. | +86-18168774353 |
boquanshengwu003@boquansw.com | China | 248 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3878 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 7193 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5872 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3002 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 300 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18629 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |