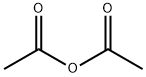
무수초산
|
|
무수초산 속성
- 녹는점
- -73.1 °C
- 끓는 점
- 140 °C
- 밀도
- 1.087
- 증기 밀도
- 3.5 (vs air)
- 증기압
- 10 mm Hg ( 36 °C)
- 굴절률
- n
20/D 1.390(lit.)
- 인화점
- 130 °F
- 저장 조건
- Store at RT.
- 용해도
- 에테르, 클로로포름, 벤젠과 혼합 가능.
- 물리적 상태
- 액체
- 색상
- 무색의
- Specific Gravity
- 1.082
- 수소이온지수(pH)
- 3 (10g/l, H2O, 20°C)
- 냄새
- 매우 강한; 날카로운; 식초 같은 특유의 냄새.
- 폭발한계
- 2.0-10.2%(V)
- ?? ??
- 산성의
- 수용성
- 물과 반응
- 감도
- Moisture Sensitive
- Merck
- 14,56
- BRN
- 385737
- Dielectric constant
- 20.0
- 노출 한도
- NIOSH REL: ceiling 5 ppm (20 mg/m3), IDLH 200 ppm; OSHA PEL: 5 ppm; ACGIH TLV: ceiling 5 ppm.
- 안정성
- 안정성 가연성. 강한 산화제, 물, 강염기, 알코올과 호환되지 않습니다.
- LogP
- -0.58 at 20℃
- CAS 데이터베이스
- 108-24-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xn,F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-20/21/22-37/38-41-34-20/21-10-20/22-19-40 | ||
| 안전지침서 | 26-39-45-36/37/39-33-16 | ||
| 유엔번호(UN No.) | UN 2924 3/PG 2 | ||
| OEL | Ceiling: 5 ppm (20 mg/m3) | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AK1925000 | ||
| F 고인화성물질 | 21 | ||
| 자연 발화 온도 | 629 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 29152400 | ||
| 유해 물질 데이터 | 108-24-7(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 1.78 g/kg (Smyth) | ||
| IDLA | 200 ppm | ||
| 기존화학 물질 | KE-00017 |
무수초산 C화학적 특성, 용도, 생산
용도
초산셀룰로즈의 제조, 유기약품의 아세틸화제, 염료, 향료, 합성수지의 제조, 아스피린, 기타 약품, 초산에스테르류, 유기합성원료, 사진필름(불연성) 등에 사용.개요
Acetic anhydride (chemical formula: (CH3CO)2O) is the simplest isolable anhydride of a carboxylic acid, is widely used as a reagent in organic synthesis. It has an internal asymmetric structure, leading to its potent electrophilicity. In organic chemistry, it is mainly used in acetylation for the manufacture of commercially significant materials, e.g. it can be used for the conversion of cellulose to cellulose acetate and aspirin. It can also be used as a wood preservative. In starch industry, it is a common acetylation compound for acetylation of monoglyceride. It is also an esterification agent for the production of modified starches. It is produced by carbonylation of methyl acetate or the reaction between ketene and acetic acid.화학적 성질
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis. It is a combustible, colorless, strongly refractive, liquid that smells strongly of acetic acid, formed by its reaction with the moisture in the air. Acetic anhydride is an esterification agent used in the preparation of modified starch and for acetylation of acetylated monoglycerides.물리적 성질
Acetic anhydride is a colorless, very mobile liquid with a very strong, acetic acid-like odor. Experimentally determined detection and recognition odor threshold concentrations were <600 μg/m3 (<140 ppbv) and 1.5 mg/m3 (360 ppbv), respectively (Hellman and Small, 1974). Acetic anhydride is miscible with polar solvents and dissolves in cold alcohol with slow decomposition. The solubility of acetic anhydride in water at 20°C is 2.6wt%, with slow decomposition; the solubility of water in acetic anhydride at 15°C is 10.7 wt%, with gradual decomposition.출처
Reported found in watercress (Nasturtium officinale r. br.).용도
Acetic anhydride is an important solvent and acetylation agent. It is used for the manufacture of acetylcellulose, acetylsalicylic acid, acetanilide, nitrofurane, sulfonamides, vitamin B6 etc. As acetulizer and solvent in examining wool fat, glycerol, fatty and volatile oils, resins; detection of rosin. Widely used in organic syntheses, e.g., as dehydrating agent in nitrations, sulfonations and other reactions where removal of water is necessary.정의
ChEBI: Acetic anhydride is an acyclic carboxylic anhydride derived from acetic acid. It has a role as a metabolite and a reagent.주요 응용
As indicated by its organic chemistry, Ac2O is mainly used for acetylations leading to commercially significant materials. Its largest application is for the conversion of cellulose to cellulose acetate, which is a component of photographic film and other coated materials. Similarly it is used in the production of aspirin (acetylsalicylic acid), which is prepared by the acetylation of salicylic acid.It is also used as a wood preservative via autoclave impregnation to make a longer lasting timber.In starch industry, acetic anydride is a common acetylation compound, used for the production of modified starches
Because of its use for the synthesis of heroin by the diacetylation of morphine, acetic anhydride is listed as a U.S. DEA List II precursor, and restricted in many other countries.
화학 반응
introduction of acetyl groups to organic substrates . In these conversions, acetic anhydride is viewed as a source of CH3CO+. Alcohols and amines are readily acetylated. For example, the reaction of acetic anhydride with ethanol yields ethyl acetate:(CH3CO)2O + CH3CH2OH → CH3CO2CH2CH3 + CH3COOH
Often a base such as pyridine is added to function as catalyst. In specialized applications, Lewis acidic scandium salts have also proven effective catalysts.
Aromatic rings are acetylated, usually in the presence of an acid catalyst. Illustrative is the conversion of benzene to aceto phenone:
(CH3CO)2O + C6H6 → CH3COC6H5 + CH3CO2H
Ferrocene can be acetylated as well:
Cp2Fe + (CH3CO)2O → CpFe(C5H4COCH3).
일반 설명
A clear colorless liquid with a strong odor of vinegar. Flash point 129°F. Corrosive to metals and tissue. Density 9.0 lb /gal. Manufacture of cellulose esters, plastics, pharmaceuticals, photographic films, cigarette filters, and magnetic tape; inorganic synthesis as an acetylating agent, bleaching agent, and dehydrating agent.공기와 물의 반응
Flammable. Reacts violently with water to generate acetic acid . This reaction is heightened by the presence of mineral acids (nitric, perchloric, sulfuric acid, etc.) [Chem. Eng. News 25, 3458].반응 프로필
Acetic anhydride reacts violently on contact with water, steam, methanol, ethanol, glycerol and boric acid. Reaction with water is particularly dangerous in presence with mineral acids (e.g., nitric, perchloric, chromic, sulfuric acid) [Chem. Eng. News 25, 3458]. Potentially explosive reactions with oxidizing reagents such as barium peroxide, chromium trioxide, chromic acid, hypochlorous acid, nitric acid, perchloric acid, peroxyacetic acid, potassium permanganate, hydrogen peroxide. [Sax, 9th ed., 1996, p. 15]. Reacts violently with metal nitrates used as nitrating agents [Davey W. et al., Chem. & Ind., 1948, p. 814].건강위험
Liquid is volatile and causes little irritation on uncovered skin. However, causes severe burns when clothing is wet with the chemical or if it enters gloves or shoes. Causes skin and eye burns and irritation of respiratory tract. Nausea and vomiting may develop after exposure.화학 반응
Reactivity with Water: Reacts slowly with water, but considerable heat is liberated when contacted with spray water; Reactivity with Common Materials: Corrodes iron, steel and other metals; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Dilute with water and use sodium bicarbonate solution to rinse; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Safety Profile
Moderately toxic by inhalation, ingestion, and skin contact. A skin and severe eye irritant. A flammable liquid. A fire and explosion hazard when exposed to heat or flame. Potentially explosive reactions with barium peroxide, boric acid, chromium trioxide, 1,3diphenyltriazene, hydrochloric acid + water, hypochlorous acid, nitric acid, perchloric acid + water, peroxyacetic acid, potassium permanganate, tetrafluoroboric acid, 4toluenesulfonic acid + water, and acetic acid + water. Reactions with ethanol + sodium hydrogen sulfate, and hydrogen peroxide form explosive products. Reactions with ammonium nitrate + hexamethylenetetrammonium acetate + nitric acid form as products the military explosives RDX and HMX. Reacts violently with N-tert-butyl-phthalimic acid + tetrafluoroboric acid, chromic acid, glycerol + phosphoryl chloride, and metal nitrates (e.g., copper or sodium nitrates). Incompatible with 2-aminoethanol, aniline, chlorosulfonic acid, (CrOs + acetic acid), ethylene-diamine, ethyleneimine, glycerol, oleum, HF, permanganates, NaOH, Na2O2, H2SO4, water, N2O2, (glycerol + phosphoryl chloride). When heated to decomposition it emits toxic fumes; can react vigorously with oxidizing materials, wdl react violently on contact with water or steam. Used in production of drugs of abuse. To fight fire, use CO2, dry chemical, water mist, alcohol foam. See also ANHYDRIDES.잠재적 노출
Acetic anhydride is used as an acetylating agent or as a solvent in the manufacture of cellulose acetate, acetanilide, aspirin, synthetic fibers, plastics, explosives, resins, perfumes, and flavorings; and it is used in the textile dyeing industry. It is widely used as a pharmaceutical intermediate and as a pesticide intermediate환경귀착
Chemical/Physical. Slowly dissolves in water forming acetic acid. In ethanol, ethyl acetate is formed (Windholz et al., 1983).운송 방법
UN1715 Acetic anhydride, Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid.Purification Methods
Adequate purification can usually be achieved by fractional distillation through an efficient column. Acetic acid can be removed by prior refluxing with CaC2 or with coarse Mg filings at 80-90o for 5days, or by distillation from a large excess of quinoline (1% AcOH in quinoline) at 75mm pressure. Acetic anhydride can also be dried by standing with Na wire for up to a week, removing the Na and distilling it under vacuum. (Na reacts vigorously with acetic anhydride at 65-70o). Dippy & Evans [J Org Chem 15 451 1950] let the anhydride (500g) stand over P2O5 (50g) for 3hours, then decanted it and stood it with ignited K2CO3 for a further 3hours. The supernatant liquid was distilled and the fraction b 136-138o was further dried with P2O5 for 12hours, followed by shaking with ignited K2CO3, before two further distillations through a five-section Young and Thomas fractionating column. The final material distilled at 137.8-138.0o. It can also be purified by azeotropic distillation with toluene: the azeotrope boils at 100.6o. After removal of the remaining toluene, the anhydride is distilled [sample had a specific conductivity of 5 x 10-9 ohm-1cm -1]. [Beilstein 2 H 96, 2 I 39, 2 II 91, 2 III 134, 2 IV 94.] Rapid procedure: Shake with P2O5, separate, shake with dry K2CO3 and fractionally distil.비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alcohols, chromic acid (violent reaction), amines, strong caustics; finely divided metals. Contact with water forms acetic acid and liberates a large amount of heat. Corrosive to iron, steel and other metals.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.무수초산 준비 용품 및 원자재
원자재
준비 용품
(2R,3R)-2-Amino-3-methylpentanoic acid
2-cyano-N-methyl-N-[(methylamino)carbonyl]acetamide
DL-THREO-2-ACETAMIDO-L-(4-니트로페닐)-L,3-프로판디올
N-Acetyl-L-glutamine
1,3-Dimethyl-8-nitro-1H-purine-2,6(3H,9H)-dione ,97%
비타민 E
아세틸리시놀산메틸에스테르
아세틸모르폴린
2-ACETYLAMINO-5-BROMO-6-METHYLPYRIDINE
마그네슘 아세트산
6-(ACETYLAMINO)-2-PYRIDINECARBOXYLIC ACID
N-(2-METHOXY-4-NITROPHENYL)ACETAMIDE
1-BENZOTHIOPHENE-3-SULFONYL CHLORIDE
4-(METHOXYCARBONYL)NICOTINIC ACID
2,4,6-트리하이드록시-1,3-디메틸벤젠
(6-BROMO-2-PYRIDINYL)-카르밤산,1,1-디메틸에틸에스테르
2-Acetylamino-4-methylpyridine
안티모니 트라이아세트산(안티몬 트리아세트산)
6-Amino-1,3-dimethyl-1,2,3,4-tetrahydropyrimidine-2,4-dione
5-알파-안드로스테인-알파-노르-2,17-디온
4-AMINO-2-(METHYLTHIO)PYRIMIDINE-5-CARBONITRILE
아세트산 셀룰로스
water-proofing agent PF
2-ACETAMIDO-3-PICOLINE
아세트산2-에틸부틸에스테르
4-Acetamido-2-chloropyridine
3,4-피리딘디카르복시마이드
7-(아세틸아미노)-4-하이드록시-2-나프탈렌설폰산
2-[(Acetyloxy)methoxy]ethyl acetate
파네솔
4-ACETAMIDO-3-NITROPYRIDINE
3-아세틸디메틸아민
19-Nor-17-alpha-pregna-3,5-dien-20-yne-3,17-diol, diacetate
2-ACETAMIDO-5-PICOLINE
메틸2-시아노-3-에톡시아크릴레이트
N-Hydroxysulfosuccinimide sodium salt
N-(6-CHLORO-3-NITROPYRIDIN-2-YL)ACETAMIDE
3-(METHOXYCARBONYL)PYRIDINE-2-CARBOXYLIC ACID
3,4-피리딘디카르복실릭 안하이드리드
D-Phenylalanine
펭지톡신