초산에틸
|
|
초산에틸 속성
- 녹는점
- −84 °C(lit.)
- 끓는 점
- 76.5-77.5 °C(lit.)
- 밀도
- 0.902 g/mL at 25 °C(lit.)
- 증기 밀도
- 3 (20 °C, vs air)
- 증기압
- 73 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.3720(lit.)
- FEMA
- 2414 | ETHYL ACETATE
- 인화점
- 26 °F
- 저장 조건
- Store at +2°C to +25°C.
- 용해도
- 에탄올, 아세톤, 디에틸 에테르 및 벤젠과 혼합 가능합니다.
- 산도 계수 (pKa)
- 16-18(at 25℃)
- 물리적 상태
- 액체
- Specific Gravity
- 0.902 (20/20℃)
- 색상
- APHA: ≤10
- 상대극성
- 0.228
- 냄새
- 7~50ppm(평균 = 18ppm)에서 감지 가능한 기분 좋은 과일 향
- 폭발한계
- 2.2-11.5%, 38°F
- Odor Threshold
- 0.87ppm
- ?? ??
- 미묘한
- 수용성
- 80g/L(20℃)
- 최대 파장(λmax)
- λ: 256 nm Amax: ≤1.00
λ: 275 nm Amax: ≤0.05
λ: 300 nm Amax: ≤0.03
λ: 325-400 nm Amax: ≤0.005
- JECFA Number
- 27
- Merck
- 14,3757
- BRN
- 506104
- Henry's Law Constant
- 0.39 at 5.00 °C, 0.58 at 10.00 °C, 0.85 at 15.00 °C, 1.17 at 20.00 °C, 1.58 at 25.00 °C (column stripping-UV, Kutsuna et al., 2005)
- 노출 한도
- TLV-TWA 400 ppm (~1400 mg/m3) (ACGIH, MSHA, and OSHA); IDLH 10,000 ppm (NIOSH).
- Dielectric constant
- 23.0(Ambient)
- 안정성
- 안정적인. 다양한 플라스틱, 강한 산화제와 호환되지 않습니다. 가연성이 높습니다. 증기/공기 혼합물은 폭발성이 있습니다. 습기에 민감할 수 있습니다.
- InChIKey
- XEKOWRVHYACXOJ-UHFFFAOYSA-N
- LogP
- 0.68-0.73 at 20-25℃
- CAS 데이터베이스
- 141-78-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xi,Xn,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-36-66-67-20/21/22-10-39/23/24/25-23/24/25-68/20/21/22 | ||
| 안전지침서 | 16-26-33-36/37-45-7-25 | ||
| 유엔번호(UN No.) | UN 1173 3/PG 2 | ||
| OEB | A | ||
| OEL | TWA: 400 ppm (1400 mg/m3) | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | AH5425000 | ||
| F 고인화성물질 | 1 | ||
| 자연 발화 온도 | 427 °C | ||
| TSCA | Yes | ||
| HS 번호 | 2915 31 00 | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 141-78-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 11.3 ml/kg (Smyth) | ||
| IDLA | 2,000 ppm [10% LEL] | ||
| 기존화학 물질 | KE-00047 | ||
| 유해화학물질 필터링 | 97-1-161 | ||
| 사고대비 물질 필터링 | 32 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 아세트산 에틸 및 이를 85% 이상 함유한 혼합물 |
초산에틸 C화학적 특성, 용도, 생산
용도
에틸 아세테이트는 용매로 가장 일반적으로 사용됩니다 (희석 특성 때문에). 또한 목재 가구, 농업, 건설 장비, 광산 장비 및 해양 용도의 코팅 제형에 사용됩니다. 이 제품의 주요 사용자 최종 시장은 전자, 화장품, 인쇄, 식품 및 코팅 산업입니다.순도시험
(1) 비중 : 이 품목의 비중은 0.897~0.906이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 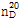 은 1.370~1.375이어야 한다.
은 1.370~1.375이어야 한다.
(3) 산가 : 이 품목 20g을 취하여 향료시험법 중 산가측정법에 따라 시험할 때, 그 값은 0.1 이하이어야 한다.
확인시험
(1) 이 품목 1mL에 수산화나트륨용액(1→4) 5mL를 가하여 수욕 중에서 흔들어 섞으면서 가열하면 특이한 방향은 없어지고 이 액을 묽은황산으로 산성으로 하여 수욕 중에서 흔들어 섞으면서 가열하면 초산의 냄새를 발생한다.
(2) 이 품목 1mL에 수산화나트륨시액 25mL를 가하여 수욕 중에서 5분간 가열하고 식힌 다음 묽은염산으로 중화하고 염화제이철시액 5방울을 가하면 진한 적색을 나타낸다.
정량법
이 품목 약 1g을 미리 에탄올 10mL를 넣은 100mL 플라스크에 넣어 정밀히 달고 0.5N 알콜성수산화칼륨용액 40mL를 가하여 환류냉각기를 달아 80±2℃의 수욕상에서 20분간 가열한 다음 식히고 0.5N 염산으로 과잉의 알칼리를 적정한다(지시약 : 페놀프탈레인시액 2~3방울). 따로 같은 방법으로 공시험을 한다.
0.5N 알콜성수산화칼륨용액 1mL = 44.06mg C4H8O2
포장, 보관 및 운송
저장 및 운송 : 저장은 일반적으로 산화제가 함유되지 않은 시원하고 건조하며 통풍이 잘되는 시설에 보관됩니다. 에틸 아세테이트는 직사 광선, 열 및 화염에 노출되지 않도록하십시오.개요
Ethyl acetate (systematically, ethyl ethanoate, commonly abbreviated EtOAc or EA) is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, decaffeinating tea and coffee, and cigarettes (see list of additives in cigarettes). Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tons. In 2004, an estimated 1.3M tons were produced worldwide.화학적 성질
Ethyl acetate has a pleasant ethereal fruity, brandy-like odor, reminiscent of pineapple, somewhat nauseating in high concentration. It has fruity sweet taste when freshly diluted in water. Ethyl acetate is probably one of the most used of all flavor chemicals by volume. Ethyl acetate is slowly decomposed by moisture and then acquires an acid status due to the acetic acid formed.물리적 성질
Clear, colorless, mobile liquid with a pleasant, sweet fruity odor. Experimentally determined detection and recognition odor threshold concentrations were 23 mg/m3 (6.4 ppmv) and 48 mg/m3 (13.3 ppmv), respectively (Hellman and Small, 1974). Cometto-Mu?iz and Cain (1991) reported an average nasal pungency threshold concentration of 67,300 ppmv.출처
Although it has been reported present in some natural fruital aromas and in some distillates (rum, rum ether), it has not been reported yet as a constituent of essential oils; it has been identified also in the petals of Magnolia fuscata. Reported found in many foods including fresh and cooked apple, apricot, banana (169 ppm), sweet and sour cherry, citrus peel oils and juices, blueberry, cranberry, black currants, raspberry, blackberry, guava, passion fruit, melon, peaches, papaya, pineapple, cabbage, onion, leek, potato, tomato (3 to 6 ppm), clove, ginger, vinegar, breads, cheeses (0.2 to 0.8 ppm), butter (2 ppm), yogurt, milk, meats, cognac, beer (4 to 64 ppm), whiskies, cider, sherry, grape wines, rum, cocoa, coffee, tea, filberts, peanuts, popcorn, oats, honey, soybeans, coconut, olive oil (0.02 ppm) and olive.용도
Ethyl acetate is used as a solvent for varnishes, lacquers, and nitrocellulose; as anartificial fruit flavor; in cleaning textiles;and in the manufacture of artificial silk andleather, perfumes, and photographic filmsand plates (Merck 1996). Ethyl Acetate is generally used as a solvent in organic reactions. Environmental contaminants; Food contaminants.제조 방법
Ethyl acetate is made by esterification of acetic acid with ethanol, from acetaldehyde, or by the direct addition of ethylene to acetic acid. BP started a 220,000 tonne/year plant in 2001 to operate the last of these processes, known as AVADA. Ethylene and acetic acid react in the presence of a heteropolyacid catalyst to give ethyl acetate at a claimed high selectivity and 99.97% purity. This is the world’s largest ethyl acetate plant and is motivated by its increasing use as a more “acceptable” solvent than hydrocarbons.In some countries, where ethanol is expensive or there is surplus acetaldehyde capacity, ethyl acetate is made by a Tishchenko reaction. Sasol in South Africa was said to be investigating such a process in the early 2000s. Ethanol is a solvent for surface coatings, cleaning preparations, and cosmetics. Industrial ethanol is aerobically fermented to white vinegar (dilute acetic acid) of the type used for pickling. Gourmet vinegars—wine vinegar, cider vinegar, and so on, made by fermentation of alcoholic beverages—are also available. Ten percent of industrial ethanol production was used for vinegar in the United States in 2001.
생산 방법
Ethyl acetate can be manufactured by the slow distillation of a mixture of ethanol and acetic acid in the presence of concentrated sulfuric acid. It has also been prepared from ethylene using an aluminum alkoxide catalyst.화학 반응
Ethyl acetate can be hydrolyzed in acidic or basic conditions to regain acetic acid and ethanol. The use of an acid catalyst accelerates the hydrolysis, which is subject to the Fischer equilibrium mentioned above. In the laboratory, and usually for illustrative purposes only, ethyl esters are typically hydrolyzed in a two step process starting with a stoichiometric amount of strong base, such as sodium hydroxide. This reaction gives ethanol and sodium acetate, which is unreactive toward ethanol:CH3CO2C2H5 + Na OH → C2H5OH + CH3CO2Na
The rate constant is 0.111 dm3 / mol.sec at 25 °C.
일반 설명
Ethyl acetate, a carboxylate ester, is bio-friendly organic solvent with wide range of industrial applications. Its synthesis by reactive distillation and by acceptorless dehydrogenative dimerization of ethanol has been explored. Its utility as a less toxic alternative to diethyl ether in the formalin-ether (F-E) sedimentation procedure for intestinal parasites has been investigated. Its ability as an acyl acceptor in the immobilized lipase-mediated preparation of biodiesel from crude vegetable oils has been examined. The complete degradation of ethyl acetate to CO2 using manganese octahedral molecular sieve (OMS-2) has been investigated.공기와 물의 반응
Highly flammable. Slightly soluble in water. Ethyl acetate is slowly hydrolyzed by moisture.반응 프로필
Ethyl acetate is also sensitive to heat. On prolonged storage, materials containing similar functional groups have formed explosive peroxides. Ethyl acetate may ignite or explode with lithium aluminum hydride. Ethyl acetate may also ignite with potassium tert-butoxide. Ethyl acetate is incompatible with nitrates, strong alkalis and strong acids. Ethyl acetate will attack some forms of plastics, rubber and coatings. Ethyl acetate is incompatible with oxidizers such as hydrogen peroxide, nitric acid, perchloric acid and chromium trioxide. Violent reactions occur with chlorosulfonic acid. . SOCl2 reacts with esters, such as Ethyl acetate, forming toxic SO2 gas and water soluble/toxic acyl chlorides, catalyzed by Fe or Zn (Spagnuolo, C.J. et al. 1992. Chemical and Engineering News 70(22):2.).건강위험
The acute toxicity of ethyl acetate is low. Ethyl acetate vapor causes eye, skin, and respiratory tract irritation at concentrations above 400 ppm. Exposure to high concentrations may lead to headache, nausea, blurred vision, central nervous system depression, dizziness, drowsiness, and fatigue. Ingestion of ethyl acetate may cause gastrointestinal irritation and, with larger amounts, central nervous system depression. Eye contact with the liquid can produce temporary irritation and lacrimation. Skin contact produces irritation. Ethyl acetate is regarded as a substance with good warning properties. No chronic systemic effects have been reported in humans, and ethyl acetate has not been shown to be a human carcinogen, reproductive, or developmental toxin인화성 및 폭발성
Ethyl acetate is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Ethyl acetate vapor forms explosive mixtures with air at concentrations of 2 to 11.5% (by volume). Hazardous gases produced in ethyl acetate fires include carbon monoxide and carbon dioxide. Carbon dioxide or dry chemical extinguishers should be used for ethyl acetate fires.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Pharmaceutical Applications
In pharmaceutical preparations, ethyl acetate is primarily used as a solvent, although it has also been used as a flavoring agent. As a solvent, it is included in topical solutions and gels, and in edible printing inks used for tablets.Ethyl acetate has also been shown to increase the solubility of chlortalidone and to modify the polymorphic crystal forms obtained for piroxicam pivalate, mefenamic acid, and fluconazole,and has been used in the formulation of microspheres. Ethyl acetate has been used as a solvent in the preparation of a liposomal amphotericin B dry powder inhaler formulation.(9) Its use as a chemical enhancer for the transdermal iontophoresis of insulin has been investigated.
In food applications, ethyl acetate is mainly used as a flavoring agent. It is also used in artificial fruit essence and as an extraction solvent in food processing.
Safety Profile
Potentially poisonous by ingestion. Toxicity depends upon alcohols in question, generally ethanol with methanol as a denaturant. A flammable liquid and dangerous fire hazard; can react vigorously with oxidzing materials. Moderate explosion hazard. See ETHANOL, METHYL ALCOHOL, and n-PROPYL ALCOHOL.Safety
Ethyl acetate is used in foods, and oral and topical pharmaceutical formulations. It is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient.However, ethyl acetate may be irritant to mucous membranes, and high concentrations may cause central nervous system depression. Potential symptoms of overexposure include irritation of the eyes, nose, and throat, narcosis, and dermatitis.
Ethyl acetate has not been shown to be a human carcinogen or a reproductive or developmental toxin.
The WHO has set an estimated acceptable daily intake of ethyl acetate at up to 25 mg/kg body-weight.
In the UK, it has been recommended that ethyl acetate be temporarily permitted for use as a solvent in food and that the maximum concentration consumed in food should be set at 1000 ppm.
LD50 (cat, SC): 3.00 g/kg
LD50 (guinea-pig, oral): 5.50 g/kg
LD50 (guinea-pig, SC): 3.00 g/kg
LD50 (mouse, IP): 0.709 g/kg
LD50 (mouse, oral): 4.10 g/kg
LD50 (rabbit, oral): 4.935 g/kg
LD50 (rat, oral): 5.62 g/kg
잠재적 노출
This material is used as a solvent for nitrocellulose and lacquer. It is also used in making dyes,flavoring and perfumery, and in smokeless powder manufactureCarcinogenicity
Ethyl acetate was not mutagenic in bacterial assays; it was not genotoxic in a number of in vivo assays but did cause chromosomal damage in hamster cells in vitro.Ethyl acetate has a fruity odor detectable at 10ppm.
The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for ethyl acetate is 400pm (1440mg/m3).
환경귀착
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.00 g/g which is 54.9% of the ThOD value of 1.82 g/g.Photolytic. Reported rate constants for the reaction of ethyl acetate and OH radicals in the atmosphere (296 K) and aqueous solution are 1.51 x 10-12 and 6.60 x 10-13 cm3/molecule?sec, respectively (Wallington et al., 1988b).
Chemical/Physical. Hydrolyzes in water forming ethanol and acetic acid (Kollig, 1993). The estimated hydrolysis half-life at 25 °C and pH 7 is 2.0 yr (Mabey and Mill, 1978).
신진 대사
Ethyl acetate is hydrolysed to ethyl alcohol, which is then partly excreted in the expired air and urine. The rest is metabolized, the acetate fraction becoming incor porated in the body pool (Fassett, 1963).저장
Ethyl acetate should be stored in an airtight container, protected from light and at a temperature not exceeding 30°C. Ethyl acetate is slowly decomposed by moisture and becomes acidic; the material can absorb up to 3.3% w/w water.Ethyl acetate decomposes on heating to produce ethanol and acetic acid, and will emit acrid smoke and irritating fumes. It is flammable and its vapor may travel a considerable distance to an ignition source and cause a ‘flashback’.
The alkaline hydrolysis of ethyl acetate has been shown to be inhibited by polyethylene glycol and by mixed micelle systems.
운송 방법
UN1173 Ethyl acetate, Hazard Class: 3; Labels: 3-Flammable liquid.Purification Methods
The most common impurities in EtOAc are water, EtOH and acetic acid. These can be removed by washing with aqueous 5% Na2CO3, then with saturated aqueous CaCl2 or NaCl, and drying with K2CO3, CaSO4 or MgSO4. More efficient drying is achieved if the solvent is further dried with P2O5, CaH2 or molecular sieves before distillation. CaO has also been used. Alternatively, ethanol can be converted to ethyl acetate by refluxing with acetic anhydride (ca 1mL per 10mL of ester), the liquid is then fractionally distilled, dried with K2CO3 and redistilled. [Beilstein 2 III 127.]비 호환성
Ethyl acetate can react vigorously with strong oxidizers, strong alkalis, strong acids, and nitrates to cause fires or explosions. It also reacts vigorously with chlorosulfonic acid, lithium aluminum hydride, 2-chloromethylfuran, and potassium tert-butoxide.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.Regulatory Status
Included in the FDA Inactive Ingredients Database (oral tablets and sustained-action tablets; topical and transdermal preparations). Included in nonparenteral medicines licensed in the UK (tablets, topical solutions, and gels). Ethyl acetate is also accepted for use in food applications in a number of countries including the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.초산에틸 준비 용품 및 원자재
원자재
준비 용품
self curing adhesive SL-B404
D-글루코스펜타키스[3,4-디히드록시-5-[(트리히드록시-3,4,5-벤조일)옥시]벤조에이트]
알루미늄 에틸아세토 아세테이트 디이소프로필레이트
adhesive JX-18-1
adhesive M-861 for polyolefin plastics
헥사데실트리메틸암모늄 염화물
ETHYL PICOLINOYLACETATE
ETHYL ISONICOTINOYLACETATE
Enoximone
N-Acetylethylenediamine
Water-proof adhesive
트리페닐실라놀
special adhesive JA-501 for laminating packaging materials
Boc-O-benzyl-L-tyrosine
2-(4-Ethoxyphenyl)-2-methylpropanol
2-Amino-6-bromopyridine
녹차추출물
3-METHYL-4-PYRIDINECARBOXYLIC ACID
N-ETHYL 3-NITROBENZENESULFONAMIDE
special adhesive JA-502 for aluminum-plastics laminating tape
wealant XY-2
2-아세틸티아졸
Methyl 4-bromo-3-nitrobenzoate
4-FLUOROBENZYL ISOCYANATE
4(1H)-Pyrimidinone, 2-methyl- (8CI,9CI)
2-(2-포르밀-페녹시)-프로피온산
adhesive No.1 for shrink packaging
3-히드록시피페리딘
암피실린 나트륨
BOC-Glycine
Diphenyl N-cyanocarbonimidate
dry laminating adhesive AD
polyurethane adhesive for dry laminating
N-BENZYL-6-CHLORO-N-METHYLPYRIDAZIN-3-AMINE
2-이소시아나토-4,6-디메톡시피리미딘
Methyl 3-bromo-4-methylbenzoate
나트륨 1-헵탄설폰산
coating adhesive tiemao 102
granular adhesive PUA
N-METHOXYCARBONYLMALEIMIDE
초산에틸 공급 업체
글로벌( 496)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 |
sales@yanshuochem.com | China | 101 | 58 |
| PT CHEM GROUP LIMITED | |
peter68@ptchemgroup.com | China | 35426 | 58 |
| AS WATER CO., LTD. | +86-18994963526 +8618994963526 |
1346073549@qq.com | United Kingdom | 176 | 58 |
| Henan Xiangduo Industry Co., Ltd. | +86-15981848961 +86-15981848961 |
sales@xiangduochem.com | China | 497 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 52927 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 |
admin@hbdangtong.com | China | 1005 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 |
sales@qdrenas.com | China | 294 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |








