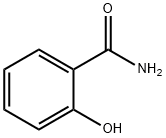
살리실아마이드 C화학적 특성, 용도, 생산
개요
Salicylamide is less acidic (pKa 8.2) than other salicylic acid derivatives. Although poorly soluble in water, stable
solutions can be formed at pH 9 through ionization of the phenolic group. It is absorbed from the GI tract on oral
administration and is rapidly metabolized to inactive metabolites by intestinal mucosa, but not by hydrolysis. Activity
appears to reside in the intact molecule. Salicylamide is approximately 40 to 55% plasma protein bound, and it
competes with other salicylates and acetaminophen for glucuronide conjugation, decreasing the extent of conjugation
of these other drugs.
화학적 성질
white or light pink crystals or powder
용도
salicylamide is an analgesic, fungicide, and anti-inflammatory ingredient used to soothe the skin. Salicylamide is an aromatic amide.
정의
ChEBI: The simplest member of the class of salicylamides derived from salicylic acid.
일반 설명
Salicylamide, o-hydroxybenzamide, is a derivative of salicylicacid that is fairly stable to heat, light, and moisture. Itreportedly exerts a moderately quicker and deeper analgesiceffect than aspirin because of quicker CNS penetration. Its metabolismdiffers from aspirin, because it is not metabolized tosalicylic acid but rather excreted exclusively as the ether glucuronideor sulfate. Thus, as a result of lack of contributionfrom salicylic acid, it has a lower analgesic and antipyretic efficacythan that of aspirin. However, it can be used in place ofsalicylates for patients with a demonstrated sensitivity to salicylates.It is also excreted much more rapidly than other salicylates,which accounts for its lower toxicity. It is available inseveral nonprescription products, in combination with acetaminophenand phenyltoloxamine (e.g., Rid-A Pain compound,Cetazone T, Dolorex, Ed-Flex, Lobac) or with aspirin,acetaminophen, and caffeine (e.g., Saleto, BC Powder).
공기와 물의 반응
Salicylamide darkens on exposure to air. . Insoluble in water.
반응 프로필
Salicylamide is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Salicylamide may be sensitive to prolonged exposure to light.
화재위험
Flash point data for Salicylamide are not available; however, Salicylamide is probably combustible.
Clinical Use
Whereas salicylamide is reported to be as effective as aspirin as an
analgetic/antipyretic and is effective in relieving pain associated with arthritic conditions, it does not appear to
possess useful anti-inflammatory activity. Thus, indications for the treatment of arthritic disease states are
unwarranted, and its use is restricted to the relief of minor aches and pain at a dosage of 325 to 650 mg three or four
times per day. Its effects in humans are not reliable, however, and its use is not widely recommended.
Purification Methods
Crystallise the amide from water or repeatedly from CHCl3 [Nishiya et al. J Am Chem Soc 108 3880 1986]. [Beilstein 10 IV 169.] The anilide [87-17-2] M 213.2, m 135o crystallises from H2O. [Beilstein 12 H 500, 12 I 268, 12 II 256, 12 944.]
살리실아마이드 준비 용품 및 원자재
원자재
준비 용품