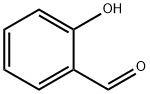
살리실 알데히드
|
|
살리실 알데히드 속성
- 녹는점
- 1-2 °C (lit.)
- 끓는 점
- 197 °C (lit.)
- 밀도
- 1.146 g/mL at 25 °C (lit.)
- 증기 밀도
- 4.2 (vs air)
- 증기압
- 1 mm Hg ( 33 °C)
- 굴절률
- n
20/D 1.573(lit.)
- 인화점
- 170 °F
- 저장 조건
- -20°C
- 용해도
- 4.9g/L
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 8.37(at 25℃)
- 색상
- 맑은 노란색
- 수소이온지수(pH)
- 6-8 (H2O, 20℃)Not applicable
- 냄새
- 쓴 아몬드
- ?? ??
- 약용
- 수용성
- 약간 용해됨
- 감도
- Air & Light Sensitive
- Merck
- 14,8326
- JECFA Number
- 897
- BRN
- 471388
- Dielectric constant
- 13.9(20℃)
- 노출 한도
- ACGIH: TWA 5 ppm (Skin)
OSHA: TWA 5 ppm(19 mg/m3)
NIOSH: IDLH 250 ppm; TWA 5 ppm(19 mg/m3); Ceiling 15.6 ppm(60 mg/m3)
- 안정성
- 안정적인. 타기 쉬운. 강염기, 강환원제, 강산, 강산화제와 호환되지 않습니다.
- InChIKey
- SMQUZDBALVYZAC-UHFFFAOYSA-N
- LogP
- 1.66 at 25℃ and pH6.2-6.3
- CAS 데이터베이스
- 90-02-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 21/22-36/38-68-36/37/38-20/21/22-51-36-51/53-22 | ||
| 안전지침서 | 26-36/37-36/37/39-36-61-64-29/35 | ||
| 유엔번호(UN No.) | 3082 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | VN5250000 | ||
| F 고인화성물질 | 8-10-23 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | II | ||
| HS 번호 | 29122990 | ||
| 유해 물질 데이터 | 90-02-8(Hazardous Substances Data) | ||
| 독성 | MLD in rats (mg/kg): 900-1000 s.c. (Binet) | ||
| 기존화학 물질 | KE-20346 |
살리실 알데히드 C화학적 특성, 용도, 생산
개요
Salicylaldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. It is a colorless or pale yellow liquid with a bitter almond odor and a burning taste. It is soluble in alcohol, benzene, and ether, and very slightly soluble in water. Salicylaldehyde is found in shrubs of the genus Spiraea and is usually produced from phenol by the action of chloroform in the presence of an alkali base. It is used in the production of coumarin, saligenin, and salicylaldoxime (an important analytical reagent), and also in analytical chemistry—for example, to detect hydrazine. Besides, salicylaldehyde is a key precursor to various chelating agents and a flavouring ingredient.화학적 성질
Salicylaldehyde is a colourless to yellow oily liquid with a pungent, irritating, bitter, almond-like odor similar to benzaldehyde, acetophenone and nitrobenzene, but with phenolic notes. It has a nut-like, coumarin flavor at low levels. slightly soluble in water, soluble in ethanol and ether. It turns purple in case of ferric chloride.출처
Occurs frequently in nature; in the flowers of Spirea ulmaria and other Spireae, in the roots of Crepis foetida L., in the fruits of Pinus avium, in the rind of Rauqolfia caffra, in the leaves of Ceanothus velutinus and in the essential oil of Cinnamomum cassia and of tobacco leaves. Also reported found in grapes, tomato, baked potato, cinnamon bark, cassia leaf, peppermint oil, pennyroyal oil, parmesan cheese, butter, milk powder, roasted chicken, beer, rum, Japanese whiskey, sherry, coffee, tea, soybean, mushroom, buckwheat, Bourbon vanilla, Chinese quince, Muscat grape, vanilla and mastic gum oil.제조 방법
Salicylaldehyde is synthesized from phenol, chloroform, and alkali according to the Reimer–Tiemman method, which was developed in 1876. starting material for the manufacture of coumarin.정의
ChEBI: Salicylaldehyde is a hydroxybenzaldehyde carrying a hydroxy substituent at position 2. It has a role as a nematicide and a plant metabolite.일반 설명
Liquid; colorless or pale yellow; bitter almond odor. Sinks and mixes slowly in water.반응 프로필
Salicylaldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.건강위험
Salicylaldehyde is a skin irritant; 500 mg/daycaused moderate irritation to rabbit skin. Itcan have injurious effects on fertility. Studieson rats indicate that subcutaneous administrationof salicylaldehyde in a high doseof >400 mg/kg can produce developmentalabnormalities, fetal death, and postimplantationmortality.The toxicity of this compound, however,is low. No toxic symptoms were noted.
LD50 value, oral (rats): 520 mg/kg
LD50 value, skin (rats): 600 mg/kg.
화재위험
Combustible. Can react with oxidizing materials.Purification Methods
It is precipitated as the bisulfite addition compound by pouring the aldehyde slowly and with stirring into a 25% solution of NaHSO3 in 30% EtOH, then standing for 30minutes. The precipitate, after filtering at the pump, and washing with EtOH, is decomposed with aqueous 10% NaHCO3, and the aldehyde is extracted into diethyl ether, dried with Na2SO4 or MgSO4, and distilled, under reduced pressure. Alternatively, salicylaldehyde is precipitated as its Cu complex by adding it to warm, saturated aqueous Cu(OAc)2, shaking and standing in ice. The precipitate is filtered off, washed with EtOH, then Et2O, and decomposed with 10% H2SO4; the aldehyde is extracted into Et2O, dried and vacuum distilled. It was also purified by dry column chromatography on Kieselgel G [Nishiya et al. J Am Chem Soc 108 3880 1986]. The acetyl derivative has m 38-39o (from pet ether or EtOH) and b 142o/18mm, 253o/atm. [Beilstein 8 IV 176.] The oxime, [94-67-7] M 137.1, crystallises CHCl3/pet ether (b 40-60o) with m 57o [Beilstein 8 IV 203.]폐기물 처리
Salicylaldehyde is burned in a chemicalincinerator equipped with an afterburner andscrubber.참고 문헌
- https://en.wikipedia.org/wiki/Salicylaldehyde
- https://pubchem.ncbi.nlm.nih.gov/compound/salicylaldehyde#section=Top
- http://www.wisegeek.com/what-is-salicylaldehyde.htm
- https://www.merriam-webster.com/medical/salicylaldehyde
- http://encyclopedia2.thefreedictionary.com/Salicylaldehyde
살리실 알데히드 준비 용품 및 원자재
원자재
준비 용품
살리실알독심
코우마린 7
2-메틸벤조푸란
메틸1-벤조푸란-2-카르복실레이트
에폭시벤즈알데히드(2-)
L-Phenylglycine
3-메틸-크롬-2-원
코우마린
5-(2-ETHOXYPHENYL)-1-METHYL-3-N-PROPYL-1,6-DIHYDRO-7H-PYRAZOLO[4,3-D]-7-PYRIMIDINONE
a copper chelate
1-BENZOFURAN-2-CARBOXAMIDE
3-ACETAMIDOCOUMARIN
Cefixime
D(-)-allo-Threonine
디옥사벤조포스
2-벤조푸란카르복실산,에틸에스테르
Amiodarone
1,3,3-트리메틸린돌리노벤조피릴로스피란
5-클로로살리실알데히드
부니트로롤
4-히드록시벤즈알데히드
2-(2-포르밀-페녹시)-프로피온산
2-[(2,6-DICHLOROBENZYL)OXY]벤잘데하이드
벤조푸란-2-카르보닐염화물
2-벤조푸란카르복스알데히드
6-메틸 벤조피론
벤조퓨란
2-메톡시벤즈알데히드
2-히드록시벤조니트릴
3-ALLYLSALICYLALDEHYDE
살리실 알데히드 공급 업체
글로벌( 508)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 |
marketing1@neostarunited.com | China | 9205 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18628 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9357 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 |
info@royal-chem.com | China | 114 | 58 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |