티오펜 C화학적 특성, 용도, 생산
화학적 성질
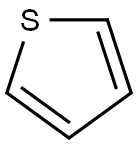
Thiophene is a colourless to pale yellow liquid that has an odor similar to benzene. It is a heterocyclic compound with a five-membered ring containing four carbon atoms and one sulfur atom. The ring system is also known as a thienyl ring. It occurs as an impurity in commercial benzene and is used as a solvent and in organic syntheses.
물리적 성질
Clear, colorless liquid with an aromatic odor resembling benzene. An odor threshold concentration
of 0.056 ppb
v was reported by Nagata and Takeuchi (1990).
용도
Thiophene is used as a building block of various organic molecules and pharmaceuticals providing functional properties.
정의
ChEBI: Thiophene is a monocyclic heteroarene that is furan in which the oxygen atom is replaced by a sulfur. It has a role as a non-polar solvent. It is a mancude organic heteromonocyclic parent, a member of thiophenes, a monocyclic heteroarene and a volatile organic compound.
생산 방법
Thiophene is present in coal tar and is recovered in the benzene distillation fraction (up to about 0.5% of the benzene present). Its removal from benzene is accomplished by mixing with concentrated sulfuric acid, soluble thiophene sulfonic acid being formed. Thiophene gives a characteristic blue coloration with isatin in concentrated sulfuric acid. The basic nomenclature of the thiophene ring system and its derivatives is indicated by the following: the sulfur atom is number 1, positions 2 and 5 are equivalent in the parent ring, as are the 3 and 4 positions.
일반 설명
Thiophene appears as a colorless liquid with an unpleasant odor. Insoluble in water and slightly denser than water. Flash point 30 °F. Vapors heavier than air. Irritates the skin, eyes, and mucous membranes. Used to make pharmaceuticals and dyes.
공기와 물의 반응
Highly flammable. Insoluble in water.
반응 프로필
Thiophene reacts violently with strong oxidizing agents and concentrated nitric acid causing fire and explosion hazards [Handling Chemicals Safely 1980. p. 899]. A mixture of Thiophene and N-nitrosoacetanilide exploded at 0°C [Ber., 1887, 30, 367].
위험도
Flammable, dangerous fire risk.
건강위험
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
화재위험
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Poison by ingestion and
intraperitoneal routes. Mildly toxic by
inhalation and subcutaneous routes. A very
dangerous fire hazard when exposed to heat
or flame. Explosive reaction with N-nitrosoacetanilide.
Violent or explosive
reaction with nitric acid. Incompatible with
oxidizing materials. To fight fire, use foam,
CO2, dry chemical. When heated to
decomposition it emits highly toxic fumes of
SOx.
환경귀착
Photolytic. A rate constant 9.70 x 10
-12 cm
3/molecule?sec was reported for the reaction of
thiophene and OH radicals in the atmosphere at room temperature (Atkinson, 1985). Thiophene
also reacts with NO3 radicals in the atmosphere at rate constants ranging from 3.2 x 10
-14
(Atkinson et al., 1985) to 3.93 x 10
-14 cm
3/molecule?sec (Atkinson, 1991).
Purification Methods
The simplest purification procedure is to dry thiophen with solid KOH, or reflux it with sodium, and fractionally distil it through a glass-helices-packed column. More extensive treatments include an initial wash with aqueous HCl, then water, drying with CaSO4 or KOH, and passage through columns of activated silica gel or alumina. Fawcett and Rasmussen [J Am Chem Soc 67 1705 1945] washed thiophene successively with 7M HCl, 4M NaOH, and distilled water, dried with CaCl2 and fractionally distilled it. *Benzene was removed by fractional crystallisation by partial freezing, and the thiophene was degassed and sealed in Pyrex flasks. [Also a method is described for recovering the thiophene from the *benzene-enriched portion.] [Beilstein 17 H 29, 17 I 17, 17 II 35, 17 III/IV 234, 17/1 V 297.]
티오펜 준비 용품 및 원자재
원자재
준비 용품