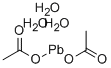
초산납
|
|
초산납 속성
- 녹는점
- 75 °C (dec.)(lit.)
- 끓는 점
- 280°C
- 밀도
- 2,55 g/cm3
- 저장 조건
- 2-8°C
- 용해도
- 443g/l
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 2.55
- 색상
- 흰색 또는 무색, 풍화성
- 수소이온지수(pH)
- 5.5-6.5 (50g/l, H2O, 20℃)
- 수용성
- 625g/L
- Hydrolytic Sensitivity
- 0: forms stable aqueous solutions
- Merck
- 14,5397
- BRN
- 3730298
- 노출 한도
- NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3
- InChIKey
- DYRQFRHCGBQJII-UHFFFAOYSA-M
- CAS 데이터베이스
- 6080-56-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 61-33-48/22-50/53-62 | ||
| 안전지침서 | 53-45-60-61 | ||
| 유엔번호(UN No.) | UN 1616 6.1/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | OF8050000 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29152900 | ||
| 독성 | LD50 orally in Rabbit: 4665 mg/kg | ||
| 유해화학물질 필터링 | 97-1-9 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 사산화납(lead tetraoxide), 황산납(lead sulfate), 염기성탄산납(basic lead carbonate)을 제외한 납화합물(Lead compounds) 및 이를 25% 이상 함유한 혼합물. 다만, 초산납(lead acetate), 알킬화납(lead alkyls), 아지드화납(lead azide), 이초산납(lead di(acetate)), 메탄술폰산납(lead(Ⅲ) methansulfonate), 인산납(lead phosphate(3:2)), 스티핀산납(lead styphate)의 경우는 이를 0.5% 이상 함유한 혼합물 |
초산납 C화학적 특성, 용도, 생산
화학적 성질
Lead acetate trihydrate, also known as plumbous acetate trihydrate, has the chemical formula Pb(C2H3O2)2·3H2O. It appears as a white, monoclinic crystalline solid. Upon heating it loses some of its water of crystallization, and after melting, decomposes at 200 °C. The trihydrate is highly soluble in water but insoluble in ethyl alcohol. It has an intensely sweet taste, hence it is sometimes called sugar of lead, but it is poisonous. The trihydrate is made by dissolving lead monoxide in hot dilute acetic acid solution; on cooling, large crystals separate, sometimes up to 60-cm long.용도
Lead acetate trihydrate, the usual commercial form, is used in the preparation of basic lead carbonate and lead chromate, as a mordant in cotton dyes, as a reagent for the manufacture of lead salts of higher fatty acids, as a water repellant, as a component in combined toning and fixing baths for daylight printing papers, and as a means of treating awnings and outdoor furniture to prevent removal of mildew- and rotproofing agents by rain or laundering. Other uses include preparation of rubber antioxidants; processing agent in the cosmetic, perfume, and toiletry industries; component of coloring agents for adhesives; and preparation of organic lead soaps as driers of paints and inks.주요 응용
Lead(II) acetate trihydrate was a component of the solution which was used towards the fabrication of platinum black microelectrodes. Lead acetate trihydrate may be used in the manufacturing of other lead salts. It may be used in the textile industry for dyeing and printing. In paint industry, lead acetate acts as a drying agent and as a purification agent in chemical synthesis.일반 설명
Lead acetate trihydrate appears as white powder or white chunky solid. Slight odor. pH (5% aqueous solution at 77°F) 5.5-6.5. It is water soluble and stable under normal conditions.공기와 물의 반응
Slowly effloresces in air. Water soluble but solutions absorb carbon dioxide from the air and to give incompletely soluble products.반응 프로필
Lead acetate trihydrate may react with acids and oxidizing agents. Is incompatible with sulfates, citrates, tartrates, chlorides, carbonates, alkalis, tannin, resorcinol, salicylic acid, phenol, chloral hydrate, sulfites, vegetable infusions and tinctures. Incompatible with phosphates . Incompatible with KBrO3 and with EDTA.화재위험
Literature sources indicate that Lead acetate trihydrate is nonflammable.Purification Methods
Crystallise it twice from anhydrous acetic acid and dry it under vacuum for 24hours at 100o. The solubility in H2O is 63% (at ~20o) and 200% (at boiling point). [Beilstein 2 IV 118.]초산납 준비 용품 및 원자재
원자재
준비 용품
초산납 공급 업체
글로벌( 333)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Shanghai Yunao International Trade Co., Ltd | +8617621705551 |
asdf@shanghaihg.cn | China | 265 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| Hebei Brisk New Material Technology Co. , Ltd. | +8613393045540 |
yilia@brisknm.com | China | 100 | 58 |
| Shijiazhuang Jianxin Biotechnology Co., LTD | +undefined+86-17692708569 |
liyingjian1997@gmail.com | China | 787 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 6710 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | +86-86-18838029171 +8618126314766 |
ada@sh-teruiop.com | China | 893 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Nanjing Deda New Material Technology Ltd. | +8613223281135 |
niki@njdeda.com | China | 76 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |