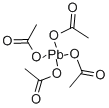
납 테트라아세트산
|
|
납 테트라아세트산 속성
- 녹는점
- 175-180 °C
- 끓는 점
- 118.1°C
- 밀도
- 2,28 g/cm3
- 저장 조건
- Inert atmosphere,2-8°C
- 용해도
- 에 용해됨클로로포름, 메탄올 (약간 용해됨)
- 물리적 상태
- 결정성 분말
- Specific Gravity
- 2.28
- 색상
- 흰색에서 밝은 주황색-분홍색
- 수용성
- 분해
- 감도
- Moisture Sensitive
- Hydrolytic Sensitivity
- 7: reacts slowly with moisture/water
- Merck
- 14,5423
- BRN
- 3595640
- 노출 한도
- NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3
- 안정성
- 안정적인. 수분에 민감합니다. 물, 대부분의 금속과 호환되지 않습니다.
- InChIKey
- JEHCHYAKAXDFKV-UHFFFAOYSA-J
- CAS 데이터베이스
- 546-67-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,O | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 61-8-20/22-33-35-50/53-62 | ||
| 안전지침서 | 53-45-60-61-36/37/39-26-17 | ||
| 유엔번호(UN No.) | UN 2923 8/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AI5300000 | ||
| F 고인화성물질 | 10-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29152900 | ||
| 유해 물질 데이터 | 546-67-8(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-21946 | ||
| 유해화학물질 필터링 | 97-1-9 | ||
| 중점관리물질 필터링 | 별표2-67 | ||
| 암, 돌연변이성물질 필터링 | 296 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 사산화납(lead tetraoxide), 황산납(lead sulfate), 염기성탄산납(basic lead carbonate)을 제외한 납화합물(Lead compounds) 및 이를 25% 이상 함유한 혼합물. 다만, 초산납(lead acetate), 알킬화납(lead alkyls), 아지드화납(lead azide), 이초산납(lead di(acetate)), 메탄술폰산납(lead(Ⅲ) methansulfonate), 인산납(lead phosphate(3:2)), 스티핀산납(lead styphate)의 경우는 이를 0.5% 이상 함유한 혼합물 |
납 테트라아세트산 C화학적 특성, 용도, 생산
개요
Lead (IV) acetate or lead tetraacetate is a chemical compound with chemical formula Pb(C2H3O2)4 and is a lead salt of acetic acid. It is commercially available often stabilized with acetic acid.화학적 성질
Lead tetraacetate (plumbic acetate), Pb(C2H3O2)4, is a colorless, monoclinic crystalline solid that is soluble in chloroform and in hot acetic acid, but decomposes in cold water and in ethyl alcohol. Lead tetraacetate can be prepared by adding warm, water-free, glacial acetate acid to red lead, Pb3O4, and subsequent cooling. The salt decomposes with the addition of water to give PbO2, but the yield can be improved by passing in chlorine gas. Lead tetraacetate is available in laboratory quantities as colorless to faintly pink crystals stored in glacial acetic acid.용도
Oxidation with lead tetraacetate is often used in organic syntheses, because the lead salt is highly selective in the splitting of vicinal glycols. The rate of oxidation of cis glycols is more rapid than of the trans isomers, a property widely used in the structural determination of sugars and other polyols. Lead tetraacetate readily cleaves α-hydroxy acids as oxalic acid at room temperature. Another use is the introduction of acetoxy groups in organic molecules, as in the preparation of cyclohexyl acetate and the acetoxylation of cyclohexanol. At high temperature, methylation takes place. In these reactions, the organic molecule must contain double bonds or activating substituents.제조 방법
Lead tetraacetate can be prepared by reaction of red lead with acetic acid The other main lead acetate is lead (II) acetate.정의
ChEBI: An acetate salt with formula Pb(OAc)4. It is used as a selective oxidising agent in organic synthesis.주요 응용
Lead tetraacetate is a strong oxidizing agent, a source of acetyloxy groups and a general reagent for the introduction of lead into organolead compounds. Some of its many uses in organic chemistry :Acetoxylation of benzylic, allylic and α-oxygen ether C-H bonds, for example the photochemical conversion of dioxane to 1,4- dioxene through the 2-acetoxy-1,4-dioxane intermediate and the conversion of α-pinene to verbenone
* Oxidation of hydrazones to diazo compounds for example that of hexafluoroacetone hydrazone to bis(trifluoromethyl)diazomethane * Aziridine formation, for example the reaction of Naminophthalimide and stilbene
* Cleavage of 1,2-diols to the corresponding aldehydes or ketones often replacing ozonolysis, for instance the oxidation of di-nbutyl d-tartrate to n-butyl glyoxylate
* Reaction with alkenes to γ-lactones
* Oxidation of alcohols carrying a δ-proton to cyclic ethers.
* Oxidative cleavage of certain allyl alcohols in conjunction with ozone.
일반 설명
Faintly pink wet crystals with an odor of vinegar.공기와 물의 반응
Unstable in air. Reacts with water to form brown lead dioxide and acetic acid [Merck 11th ed. 1989].반응 프로필
Organometallics are strongly reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Generally highly toxic. Often react on contact with tissues to give toxic products.건강위험
Early symptoms of lead intoxication by ingestion are most commonly gastrointestinal disorders, colic, constipation, etc.; weakness, which may go on to paralysis chiefly of the extensor muscles of the wrists and less often of the ankles, is noticeable in the most serious cases. Ingestion of a large amount causes local irritation of the alimentary tract; pain, leg cramps, muscle weakness, paresthesias, depression, coma, and death may follow in 1 or 2 days. Contact causes severe irritation of eyes and can burn skin.화재위험
Behavior in Fire: Can increase the intensity of a fire when in contact with combustible material. Cool containers with plenty of water.Safety
Lead (IV) acetate may be fatal if ingested, inhaled, or absorbed through skin. It causes irritation to skin, eyes, and respiratory tract. It is a neurotoxin. It affects the gum tissue, central nervous system, kidneys, blood, and reproductive system.납 테트라아세트산 준비 용품 및 원자재
원자재
준비 용품
납 테트라아세트산 공급 업체
글로벌( 262)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81148696 +8615536356810 |
1047@dideu.com | China | 3408 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 29220 | 58 |
| Alfa Chemistry | |
info@alfa-chemistry.com | United States | 2344 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | CHINA | 52867 | 58 |