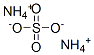황산암모늄
|
|
황산암모늄 속성
- 녹는점
- >280 °C (dec.) (lit.)
- 밀도
- 1.77 g/mL at 25 °C (lit.)
- 증기압
- <1 Pa (25 °C)
- 굴절률
- n
20/D 1.396
- 인화점
- 26 °C
- 저장 조건
- room temp
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 1.769
- 색상
- 노란색에서 주황색으로
- 수소이온지수(pH)
- 5.0-6.0 (25℃, 1M in H2O)
- 냄새
- 약간의 암모니아 냄새
- pH 범위
- 5 - 6
- 수용성
- 77g/100mL(25℃)
- 최대 파장(λmax)
- λ: 260 nm Amax: ≤0.037
λ: 280 nm Amax: ≤0.030
- Merck
- 14,555
- 안정성
- 안정적인. 강한 산화제와 접촉하면 화재나 폭발이 발생할 수 있습니다. 강한 염기와 호환되지 않습니다.
- InChIKey
- BFNBIHQBYMNNAN-UHFFFAOYSA-N
- LogP
- -5.1 at 25℃
- CAS 데이터베이스
- 7783-20-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-36/37/38-22 | ||
| 안전지침서 | 37/39-26-36-24/25 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | BS4500000 | ||
| TSCA | Yes | ||
| HS 번호 | 31022100 | ||
| 유해 물질 데이터 | 7783-20-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2840 mg/kg | ||
| 기존화학 물질 | KE-01743 |
황산암모늄 C화학적 특성, 용도, 생산
물성
• 백색의 결정으로 질소함량은 21wt%, • 암모니아와 황산이 결합된 것으로 화학적으로 중성, • 대기중에서는 녹거나 굳어지는 일이 적지만 물이나 토양 중에서는 잘 녹음, • 비효가 빠른 속효성 질소비료, • 논이나 밭 어디서나 쓸 수 있으며 기비나 추비로 다 쓸 수 있음, • 석회와 같은 알칼리성 비료와 혼합하면 기체로 변하여 질소성분의 손실을 가져옴.개요
황산 암모늄(黃酸-, ammonium sulfate)은 화학식 (NH4)2SO4을 지닌 흰 결정의 하나이다. 물에 잘 녹으며 일반적으로 토양 비료로 사용된다. 암모늄 이온 21%로서 질소, 황산염 이온 24%를 포함한다.용도
제품의권고용도질소비료.개요
암모늄 황산은 상업 사용의 번호와 무기 소금 이다. 가장 일반적인 사용은 토양 비료 . 21% 질소와 24% 황 포함 되어 있습니다. 알칼리 성 토양에 대 한 비료로 황산 암모늄의 주요 사용이 이다. 토양에서 암모늄 이온 출시 되 고 식물의 성장에 필수적인 질소를 기여 하면서 토양의 pH 균형을 낮추는 산의 작은 금액을 형성 한다. 암모늄 황산의 사용에 주요 단점은 질 산 암모늄, 운송 비용. 수용 성 살충제, 제 초 제, 살 균 제 에 대 한 농업 살포 보조로도 사용 됩니다 이르게 상대적인 낮은 질소 콘텐츠. 거기, 그것은 두 잘 물과 식물 세포에 존재 하는 철과 칼슘 양이온 바인딩 함수.용도
농업용으로는 단비와 배합비료 및 복합비료 원료로 사용되고 있으며, 공업용으로는 인견공업, 셀로판공업, 암모니아 화합물 각종 암모늄염의 제조, 효모배양, 명반제조, 피혁탄닝, 석고보드, 전구제조, 합성수지 및 의약품 원료로도 사용되고 있음순도시험
(1) 용상 : 이 품목 1g을 물 20mL에 녹일 때, 그 액은 무색으로서 탁도는 거의 징명 이하이어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(5.0ppm 이하).
(4) 셀레늄 : 이 품목 0.2g을 정밀히 달아 「황산」의 순도시험 (6)에 따라 시험한다(30ppm 이하).
확인시험
이 품목은 확인시험법 중 암모늄염 및 황산염의 반응을 나타낸다.
정량법
이 품목을 건조한 다음, 약 3g을 정밀히 달아 물에 녹여 250mL로 하고 그 중 25mL에 수산화나트륨용액(2→5) 10mL를 가하여 즉시 0.2N 황산 40mL를 넣은 수기를 접속한 증류장치에 연결하고 가열하여 암모니아를 황산 중에 유출시킨 다음 과잉의 황산을 0.2N 수산화나트륨용액으로 적정한다(지시약 : 메틸레드시액 3방울).
0.2N 황산 1mL = 13.22mg (NH4)2SO4
포장, 보관 및 운송
건조 하 고 통풍이 집에 습기에서 밀봉 패키지와 함께 저장. 해산 하 여 운송 도중 분실의 경우 비에서 자료를 방지 합니다.강열잔류물
이 품목의 강열잔류물은 0.25% 이하이어야 한다.
개요
Ammonium sulfate was the first nitrogenous fertilizer made by the Haber-Bosch process, produced by the reaction of ammonia with sulfuric acid. In contrast with the nitrate salt, it is chemically stable, not highly hygroscopic. It also supplies supplemental sulfur to soils that may be deficient in this element, but this is of minor value when it is used on soils receiving applications of ordinary superphosphate.The disadvantages of the material are its relatively low nitrogen content, which increases storage and transportation costs, and its marked tendency to cause soil acidification, which is greater than that of any other nitrogen fertilizer material.
화학적 성질
White crystalline powder물리적 성질
White crystalline solid; orthorhombic crystal; density 1.769 g/cm3 at 20°C; melts between 511 to 515°C (in a closed system): however, in an open system, it melts with decomposition at 280°C; readily dissolves in water (solubility, 70.6 g and 104 g per 100 g water at 0°C and 100°C, respectively); insoluble in acetone, alcohol and ether.출처
Ammonium sulfate occurs in trace concentrations in the upper atmosphere. It is widely used as a fertilizer for rice and other crops. It is a source of sulfur for the soil. It is also used as an additive to supply nutrient nitrogen in fermentation processes (e.g., yeast production from molasses). It also is used for fireproofing timber and plastics, and in treatment of hides, and leather production.용도
Ammonium Sulfate is a dough conditioner, firming agent, and pro- cessing aid which is readily soluble in water with a solubility of approximately 70 g in 100 g of water at 0°c. the ph of a 0.1 molar solution in water is approximately 5.5. it is used in caramel produc- tion and as a source of nitrogen for yeast fermentation. in bakery products, up to 0.25 part per 100 parts by weight of flour is used.정의
ammonium sulphate: A whiterhombic solid, (NH4)2SO4; r.d. 1.77;decomposes at 235°C. It is very solublein water and insoluble in ethanol.It occurs naturally as the mineralmascagnite. Ammonium sulphatewas formerly manufactured from the‘ammoniacal liquors’ produced duringcoal-gas manufacture but is nowproduced by the direct reaction betweenammonia gas and sulphuricacid. It is decomposed by heating torelease ammonia (and ammoniumhydrogensulphate) and eventuallywater, sulphur dioxide, and ammonia.Vast quantities of ammoniumsulphate are used as fertilizers.생산 방법
Ammonium sulfate is a high-tonnage industrial chemical, but frequently may be considered a byproduct as well as intended end-product of manufacture. A significant commercial source of (NH4)2SO4 is its creation as a byproduct in the manufacture of caprolactam, which yields several tons of the compound per ton of caprolactam made. Ammonium sulfate also is a byproduct of coke oven operations where the excess NH3 formed is neutralized with H2SO4 to form (NH4)2SO4. In the Meresburg reaction, natural or byproduct gypsum is reacted with ammonium carbonate: CaSO4·2H2O + (NH4)2CO3 CaCO3 + (NH4)2SO4 +2 H2O The product is stable, free-flowing crystals. As a fertilizer, (NH4)2SO4 has the advantage of adding sulfur to the soil as well as nitrogen. By weight, the compound contains 21% N and 24% S. Ammonium sulfate also is used in electric dry cell batteries, as a soldering liquid, as a fire retardant for fabrics and other products, and as a source of certain ammonium chemicals.일반 설명
White odorless solid. Sinks and dissolves in water.공기와 물의 반응
Dissolves in water with evolution of some heat.반응 프로필
Ammonium sulfate is acidic in aqueous solution. When a little Ammonium sulfate is added to fused potassium nitrite, a vigorous reaction occurs attended by flame [Mellor 2:702 1946-47].Safety Profile
Moderately toxic by several routes. Human systemic effects by ingestion: hypermotility, diarrhea, nausea or vomiting. See also SULFATES. Incandescent reaction on heating with potassium chlorate. Reaction with sodmm hypochlorite gves the unstable explosive nitrogen trichloride. Incompatible with (K + NH4NO3), KNO2, (NaK + NH4NO3). When heated to decomposition it emits very toxic fumes of NOx, NH3, and SOx.Purification Methods
Crystallise it twice from hot water containing 0.2% EDTA to remove metal ions, then finally from distilled water. Dry it in a desiccator for 2 weeks over Mg(ClO4)2. After 3 recrystallisations, ACS grade had Ti, K, Fe, Na at 11, 4.4, 4.4, 3.2 ppm respectively.황산암모늄 준비 용품 및 원자재
원자재
준비 용품
Thymosin α1
Compound fertilizer
알루미늄 칼륨 황산염, 도데카수화물
리파아제
네오마이신 황산
알돌라아제, 프럭토스 이인산염
PHASEOLUS VULGARIS AGGLUTININ
Nitrogen phosphorus potassium mixed fertilizer
L-(+)-아이소루신
리소자임
Ethyl 3-bromo-2-(bromomethyl)propionate
Polyinosinic acid-polycytidylic acid
PKC-GAMMA
2-AMINO-3-CYANO-4-CHLORO-5-FORMYLTHIOPHENE
아미노아실라제
리보헥산 A
페로시안화 제2철
α-Chymotrypsin
알파-아밀라아제
Lincomycin hydrochloride monohydrate
L-라이신1염산염
황산 암모늄 제1철
히알루로니다제
Bromelain
제1인산암모늄
Zirconium dicarbonate
암모늄명반
라이신
피로시다제
융모성성선자극호르몬
황산니켈암모늄
알루미늄 암모늄 황산염
3-Bromo-2-(bromomethyl)propionic acid
페니실린 감마-일라디산염 세포
카타라세
(S)-(+)-2-하이드록시-3-메틸부티르산
알코올 탈수소효소
피루브산키나제
암모늄 철 황산염
디암모늄마그네슘비스(황산염)
황산암모늄 공급 업체
글로벌( 773)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 645 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 255 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5873 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20291 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 |
admin@juchuangchem.com | China | 387 | 58 |