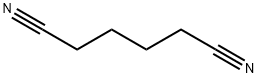
아디포니트릴
|
|
아디포니트릴 속성
- 녹는점
- 1-3 °C (lit.)
- 끓는 점
- 295 °C (lit.)
- 밀도
- 0.951 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.7 (vs air)
- 증기압
- 0.01 mm Hg ( 40 °C)
- 굴절률
- n
20/D 1.438(lit.)
- 인화점
- 163 °C
- 저장 조건
- Store below +30°C.
- 용해도
- 50g/L
- 물리적 상태
- 액체
- Specific Gravity
- 0.951
- 색상
- 무색투명~미황색
- 폭발한계
- 7-14%(V)
- 수용성
- 90g/L(20℃)
- 어는점
- 1℃
- BRN
- 1740005
- 노출 한도
- TLV-TWA 18 mg/m3 (4 ppm) (NIOSH).
- Dielectric constant
- 32.5(Ambient)
- CAS 데이터베이스
- 111-69-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 25-36/37/38-36/38-23/25 | ||
| 안전지침서 | 26-36-45-39 | ||
| 유엔번호(UN No.) | UN 2205 6.1/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | AV2625000 | ||
| 자연 발화 온도 | 460 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29269090 | ||
| 유해 물질 데이터 | 111-69-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 138 mg/kg LD50 dermal Rabbit 2134 mg/kg | ||
| 기존화학 물질 | KE-00274 |
아디포니트릴 C화학적 특성, 용도, 생산
화학적 성질
Adiponitrile is a combustible, colorless transparent to yellow, oily liquid with a slight bitter taste. Soluble in methanol, ethanol, chloroform. Insoluble in water, cyclohexane, ether, carbon disulfide and carbon tetrachloride. It decomposes on heating to react violently with strong oxidants. Upon burning, the highly toxic hydrogen cyanide is produced.용도
Adiponitrile is mainly used in the production of hexamethylenediamine for manufacturing nylon 6,6. Lesser uses include that in organic synthesis and in the preparation of adipoguanamine, which is used as an extractant for aromatic hydrocarbons. This chemical is an important intermediate for the manufacture of synthetic fiber.제조 방법
The main method of industrial production of adiponitrile is the amination of adipic acid. Adipic acid and excess ammonia are reacted in the presence of catalyst phosphoric acid or its salts or esters at a temperature of 270-290°C to generate diammonium adipate, which is then heated and dehydrated to generate crude adiponitrile, The product is obtained by rectification.생산 방법
Adiponitrile may be prepared by reacting butadiene with hydrogen cyanide, by the electrodimerization of acrylonitrile, by heating adipamide with acetic anhydride in the presence of cobalt or by reacting 1,4-dichlorobutane with sodium cyanide (HSDB 1988). Impurities such as propionitrile, bis (cyanoethyl) ether or acrylonitrile may be present depending on the method of manufacture (Smiley 1981).일반 설명
Adiponitrile appears as a colorless to light yellow liquid which is fairly soluble and is less dense than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.공기와 물의 반응
Insoluble in water.반응 프로필
1,4-Dicyanobutane is incompatible with strong oxidizers. 1,4-Dicyanobutane is also incompatible with strong acids, strong bases and strong reducing agents. .건강위험
The acute toxicity of adiponitrile is somewhat lower than that of malononitrile. It is toxic by inhalation and oral routes. Inhalation of its vapors can cause nausea, vomiting, respiratory tract irritation, and dizziness. The symptoms are similar to those of other aliphatic mono- and dinitriles. Similar poisoning effects may be manifested from ingestion of this compound. However, its toxicity is very low from skin absorption.Short et al. (1990) reported mortality and reduced weight gain in rats within one week after exposed to adiponitrile at 493 mg/m3. However, at an exposure level of 99 mg/m3 for 13 weeks the animals showed the sign of slight anemia but no histopathological evidence of organ toxicity.
LC50 value, inhalation (rats): 1710 mg/m3/ 4 hr
LD50 value, oral (mice): 172 mg/kg
There is no report of its teratogenicity or cancer-causing effects in animals or humans.
화재위험
Combustion products may contain hydrocyanic acid (HCN). Vapor may explode if ignited in an enclosed area. When heated to decomposition, 1,4-Dicyanobutane emits highly toxic fumes. Avoid oxidizing material. Hazardous polymerization may not occur.공업 용도
Adiponitrile is used in nylon manufacturing, synthetic fiber synthesis, and in the manufacture of rubber accelerators and corrosion inhibitors. It is also used as an extractant for aromatic hydrocarbons (Smiley 1981).Safety Profile
Poison by inhalation, ingestion, subcutaneous, and intraperitoneal routes. The nitrile group wdl behave as a cyanide when ingested or absorbed in the body. It produces disturbances of the respiration and circulation, irritation of the stomach and intestines, and loss of weight. Its low vapor pressure at room temperature makes exposure to harmful concentrations of its vapors unlikely if handled with Flammable when exposed to heat or flame. When heated to decomposition it emits toxic fumes of CN-. Can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. See also HYDROCYANIC ACID and NITRILESreasonable care in well-venulated areas.잠재적 노출
Is used to manufacture corrosion inhibitors, rubber accelerators, and Nylon 66; and in organic synthesis.환경귀착
The mechanism of adiponitrile’s toxicity relates to its ability to release cyanide both in vitro and in vivo. Cyanide then forms a stable complex with ferric iron in the cytochrome oxidase enzyme. Since this enzyme occupies a central role in the utilization of oxygen in practically all cells, inhibition produces an inhibition of cellular respiration.신진 대사
Animal studies indicated that the concentrations of thiocyanate in the blood and urine of guinea pigs injected with adiponitrile were proportional to the doses administered. Following administration of adiponitrile, 79% was eliminated as thiocyanate in the urine of guinea pigs (H?rtung 1982). Of the cyanide antidotes, thiosulfate was most effective in protecting against adiponitrile poisoning, and nitrite was less effective. However, on the basis of the ratio between administered adiponitrile dose and quantity of cyanide detected, Ghiringhelli, (1955) concluded that a greater part of the dose was metabolized to cyanide.운송 방법
UN2205 Adiponitrile, Hazard Class: 6.1; Labels: 6.1-Poisonous materialsPurification Methods
Reflux adiponitrile over P2O5 and POCl3, and fractionally distil it, then fractionate it through an efficient column. The liquid is TOXIC and is an IRRITANT. [Braun & Rudolph Chem Ber 67 1770 1934, Reppe et al. Justus Liebigs Ann Chem 596 127 1955, Gagnon et al. Can J Chem 34 1662 1956, Copley et al. J Am Chem Soc 62 228 1940, Beilstein 2 IV 1947.]비 호환성
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause violent reactions: fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Also incompatible with strong reducing agents such as hydrideds and active metals. Permissible Exposure Limits in Air폐기물 처리
Add excess alcoholic KOH. Than evaporate alcohol and add calcium hypochlorite. After 24 hours, flush to sewer with water. Can also be incinerated with afterburner and scrubber to remove nitrogen oxides.아디포니트릴 준비 용품 및 원자재
원자재
아디핀산
1,3-부타디엔
루테늄
TETRAETHYLAMMONIUM P-TOLUENESULFONATE
아크릴로나이트릴
암모니아(가스)
Cyclobutanecarbonitrile
사이안화 나트륨
5-Chlorovaleronitrile
1,4-디클로로부탄
준비 용품
아디포니트릴 공급 업체
글로벌( 210)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
아디포니트릴 관련 검색:
아이소부탄 에틸 시아노아크릴산 메틸렌 티오사이아네이트 부탄 염화 이소뷰틸 메틸(2-)글루타로니트릴 아르곤 1,2-디브로모-2,4-디시아노부탄 아디포니트릴
1,2-DIBROMO-2,4-DICYANOBNTANE
1,4-Dicyanobutane-d8, Hexanedinitrile-d8,1,4-dicyanobutane-d8
1,2-DICYANOCYCLOBUTENE
OCTAFLUOROADIPONITRILE
CYCLOHEXANE-1 4-DICARBONITRILE 98% MIXT&
1,3,6-HEXANETRICARBONITRILE
TRANS-CYCLOBUTANE-1,2-DICARBONITRILE
CYCLOBUTANE-1,2-DICARBONITRILE
CIS-CYCLOBUTANE-1,2-DICARBONITRILE