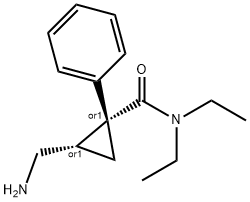
MILNACIPRAN HYDROCHLORIDE
|
|
MILNACIPRAN HYDROCHLORIDE 속성
- 끓는 점
- 393.0±21.0 °C(Predicted)
- 밀도
- 1.077±0.06 g/cm3(Predicted)
- 저장 조건
- 2-8°C
- 용해도
- H2O: 19 mg/mL
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 10.36±0.29(Predicted)
- 색상
- 하얀색
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 그림문자(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||||||||||||||||
| 예방조치문구: |
|
MILNACIPRAN HYDROCHLORIDE C화학적 특성, 용도, 생산
개요
Ixel was launched in France as an antidepressant. There are several synthetic routes, the shortest of which is five steps using benzyl cyanide as the starting material. It is a specific serotonin and noradrenaline reuptake inhibitor (SNRI). This dual mechanism of action makes it superior to selective serotonin reuptake inhibitors (SSRI) like fluoxetine and fluvoxamine. Ixel has no significant effect on postsynaptic receptors, very limited effect on cardiac function, and no quinidine-like arrhythmal effects. It has a good side effect profile with lower incidence of anticholinergic-like side effects, less sedation due to histamine H1-receptor binding, and a lack of α1- adrenoceptor antagonism. Ixel has a short half-life (7 hr) with no active metabolites. It is not metabolized by CYP450 therefore drug interaction is unlikely. It is superior in the treatment of serious depression with no need to titrate drug dose.Mechanism of action
Milnacipran selectively inhibits the reuptake of 5-HT (selectivity ratio, 9) at the presynaptic membrane site, thus increasing the concentration of 5-HT in the synaptic cleft. Although milnacipran is not a TCA, its mechanism of action is similar to that of imipramine, and its binding and reuptake inhibition profile more closely resembles that of the TCAs. Milnacipran has weak affinity for adrenergic, muscarinic, and H1 receptors and, therefore, is expected to be devoid of the prominent side effects observed for the TCAs. In clinical studies, milnacipran showed antidepressant efficacy similar to that of TCAs and SSRIs.Pharmacokinetics
In humans, milnacipran distinguishes itself from many other antidepressants by its simple pharmacokinetics. It is rapidly absorbed, with a high oral bioavailability, and it exhibits linear pharmacokinetics over a dose range of 25 to 200 mg/day. It circulates in the blood and distributes in the body principally as unmetabolized drug. Steady-state plasma levels are reached within 32 to 48 hours after twice-daily oral administration, and its metabolism does not involve the CYP enzyme system. Approximately 50% of the dose is excreted in urine as unmetabolized drug, and another 14% is excreted as its N-glucuronide conjugate. The remaining eliminated drug is composed of conjugated Phase I inactive metabolites. Because the unmetabolized drug is the only compound responsible for the activity of milnacipran, no dosage adjustment is needed in patients presenting liver impairment.Clinical Use
(±)-Milnacipran is the ci s-aminomethyl derivative of phenylcyclopropanecarboxamide that acts by inhibiting both NE and 5-HT reuptake. It is structurally different from the other NSRIs and currently is only available in Europe as a racemic mixture, with both enantiomers exhibiting antidepressant activity. Substituting the aminomethyl group of milnacipran with an aminopropyl gives a milnacipran homologue that exhibits antidepressant activity as a potent N-methyl-D-aspartate (NMDA) receptor antagonist. A glutamate hypothesis is being investigated as an alternative mechanism of depression.부작용
Milnacipran has proven to be a very safe drug, with an adverse-event profile clearly superior to that of TCAs and, to a certain extent, that of SSRIs. Only approximately 10% of patients experience side effects, and only dysuria occurred more frequently (2%) with milnacipran than with TCAs or SSRIs. Milnacipran therefore appears to be an antidepressant with a very favorable benefit:risk ratio, although with a slower onset of action than the TCAs.MILNACIPRAN HYDROCHLORIDE 준비 용품 및 원자재
원자재
준비 용품
MILNACIPRAN HYDROCHLORIDE 공급 업체
글로벌( 90)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | CHINA | 52867 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 |
sales@sarms4muscle.com | China | 10523 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 |
sales@tnjchem.com | China | 25363 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
| Hebei Miaoyin Technology Co.,Ltd | +86-17367732028 +86-17367732028 |
kathy@hbyinsheng.com | China | 3582 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43348 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131981 | 58 |
MILNACIPRAN HYDROCHLORIDE 관련 검색:
Levomilnacipran
Aniline hydrochloride
Trimethylamine hydrochloride
Betaine hydrochloride
Methylamine hydrochloride
Dimethylamine hydrochloride
Imipramine hydrochloride
Maprotiline
ILOPERIDONE
Famciclovir
Blonanserin
AGOMELATINE
(1R,2S)-rel-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride
cis-2-[(1,3-Dihydro-1,3-dioxo-2H-isoindol-2-yl)methyl-N,N-diethyl-1-phenylcyclopropanecarboxamide
MILNACIPRAN HYDROCHLORIDE
RAC CIS MILNACIPRAN-D10, HYDROCHLORIDE
cyclopropyl(phenyl)methanamine
1-Phenylcyclopropane-1-carbaldehyde