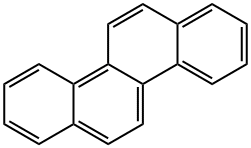
크리센
|
|
크리센 속성
- 녹는점
- 252-254 °C (lit.)
- 끓는 점
- 448 °C (lit.)
- 밀도
- 1.274
- 증기압
- 4.3 at 25 °C (de Kruif, 1980)
- 굴절률
- 1.7480 (estimate)
- 인화점
- -17℃
- 저장 조건
- Store below +30°C.
- 용해도
- <0.0001g/l
- 색상
- White to Light yellow to Light orange
- 수용성
- 불용성
- Merck
- 14,2255
- BRN
- 1909297
- Henry's Law Constant
- 1.97, 6.91, 18.8, 52.3, and 118 at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al., 1998)
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제와 호환되지 않습니다.
- InChIKey
- WDECIBYCCFPHNR-UHFFFAOYSA-N
- CAS 데이터베이스
- 218-01-9(CAS DataBase Reference)
- IARC
- 2B (Vol. 92) 2010
- NIST
- Chrysene(218-01-9)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-50/53-68-40-67-66-36-11-52/53-36/37/38 | ||
| 안전지침서 | 53-45-60-61-36/37-26-16-24/25-23 | ||
| 유엔번호(UN No.) | UN 3077 9/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | GC0700000 | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 29029090 | ||
| 유해 물질 데이터 | 218-01-9(Hazardous Substances Data) | ||
| 독성 | Acute LC50 for Neanthes arenaceodentata >50 μg/L (Rossi and Neff, 1978). | ||
| 기존화학 물질 | KE-05-0357 | ||
| 중점관리물질 필터링 | 별표2-47 |
크리센 C화학적 특성, 용도, 생산
화학적 성질
Chrysene is a combustible, white (when pure), red, or blue, fluorescent crystalline solid. Odorless. Chrysene 859 Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons물리적 성질
Orthorhombic, bipyramidal plates from benzene exhibiting strong reddish-blue fluorescence under UV light용도
Chrysene may be used as an analytical reference standard for the determination of the analyte in fish bile, air particulate extracts and food samples by various chromatography techniques.일반 설명
A crystalline solid. Denser than water and insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Toxic by ingestion. Used to make other chemicals.공기와 물의 반응
Insoluble in water.반응 프로필
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as Chrysene, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.위험도
Possible carcinogen.건강위험
There is very little information published onthe acute toxicity of chrysene. The oral toxicity is expected to be low. Animal studies showsufficient evidence of carcinogenicity. It produced skin cancer in animals. Subcutaneousadministration of chrysene in mice causedtumors at the site of application. Cancer-causing evidence in humans is not known. Ahistidine reversion–Ames test for mutagenicity showed positive.화재위험
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot.Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data by skin contact. Human mutation data reported. When heated to decomposition it emits acrid smoke and fumes.잠재적 노출
Almost never found by itself, chrysene is found in gasoline and diesel exhaust as well as in cigarette smoke; and in coal tar; coal tar pitch; creosote. It is used in organic synthesis.Carcinogenicity
The IARC has determined that there is limited evidence that chrysene is carcinogenic to experimental animals.ACGIH has classified chrysene as a confirmed animal carcinogen with unknown relevance to humans; a numerical threshold limit value (TLV) is not recommended.환경귀착
Biological. When chrysene was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, significant biodegradation with varied adaptation rates was observed. At concentrations of 5 and 10 mg/L, 59 and 38% biodegradation, respectively, were observed after 28 d (Tabak et al., 1981).Soil. The reported half-lives for chrysene in a Kidman sandy loam and McLaurin sandy loam are 371 and 387 d, respectively (Park et al., 1990).
Surface Water. In a 5-m deep surface water body, the calculated half-lives for direct photochemical transformation at 40 °N latitude, in the midsummer during midday were 13 h and 68 d with and without sediment-water partitioning, respectively (Zepp and Schlotzhauer, 1979).
Photolytic. Based on structurally related compounds, chrysene may undergo photolysis to yield quinones (U.S. EPA, 1985) and/or hydroxy derivatives (Nielsen et al., 1983). The atmospheric half-life was estimated to range from 0.802 to 8.02 h (Atkinson, 1987). Behymer and Hites (1985) determined the effect of different substrates on the rate of photooxidation of chrysene using a rotary photoreactor. The photolytic half-lives of chrysene using silica gel, alumina, and fly ash were 100, 78, and 38 h, respectively.
운송 방법
UN3077 Environmentally Hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Purification Methods
Purify chrysene by chromatography on alumina from pet ether in a darkened room. Its solution in *C6H6 is passed through a column of decolorising charcoal, then crystallised by concentrating the eluate. It has also been purified by crystallising from *C6H6 or *C6H6/pet ether, and by zone refining. [Gorman et al. J Am Chem Soc 107 4404 1985]. It is freed from 5H-benzo[b]carbazole by dissolving it in N,N-dimethylformamide and successively adding small portions of alkali and iodomethane until the fluorescent colour of the carbazole anion no longer appears when alkali is added. The chrysene (and alkylated 5H-benzo[b]carbazole) separate on addition of water. Final purification is by crystallisation from ethylcyclohexane and/or from 2-methoxyethanol [Bender et al. Anal Chem 36 1011 1964]. It can be sublimed in a vacuum. [Beilstein 5 IV 2554.]비 호환성
Contact with strong oxidizers may cause fire and explosion hazard폐기물 처리
Chrysene may be destroyed by permanganate oxidation, by high-temperature incinerator with scrubbing equipment; or by microwave plasma treatment.크리센 준비 용품 및 원자재
원자재
1,2,4-트리메틸벤젠
천연아스팔탐
말레산 무수물
Polishing oil
1-Naphthalenecarboxaldehyde, 2-(2-formylphenyl)-
2-BROMOCHRYSENE
Benzene, 1,1'-(1E)-1,2-ethenediylbis[2-ethynyl-
1,2,3,4-TETRAHYDROCHRYSENE
1-(2-((4-Methoxyphenyl)ethynyl)phenyl)ethanone
2-ETHYNYLBENZALDEHYDE
노르보나디엔(2,5-)
2-Bromobenzoyl chloride
1-Iodonaphthalene
2-(TRIMETHYLSILYL)PHENYL TRIFLUOROMETHANESULFONATE
준비 용품
크리센 공급 업체
글로벌( 261)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 |
info@spring-chem.com | China | 2068 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| City Chemical LLC | 2039322489 |
sales@citychemical.com | United States | 168 | 58 |
| TianYuan Pharmaceutical CO.,LTD | +86-755-23284190 13684996853 |
sales@tianpharm.com | CHINA | 304 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 |
info@accelachem.com | United States | 19965 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |