- Zirconium Dioxide
-

- $100.00 / 1KG
-
2024-09-26
- CAS:1314-23-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 10 ton
- Zirconium dioxide
-

- $8.00 / 25kg
-
2024-09-26
- CAS:1314-23-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20000
- Zirconium dioxide
-

- $6.00 / 1kg
-
2024-09-26
- CAS:1314-23-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
|
| Product Name: | Zirconium dioxide | | Synonyms: | Zirconium(IV) oxide, Puratronic (metals basis);Zirconium(IV) oxide, Spectrographic Grade, 99.96% min (metals basis);Zirconium(IV) oxide, 99% (metals basis excluding Hf);Zirconium(IV) oxide, 99.7% (metals basis excluding Hf), Hf <75ppm;Zirconium(IV) oxide, 99+% (metals basis excluding Hf), HfO2 2%;Zirconium(IV) oxide, 99.5% (metals basis excluding Hf), Hf <100ppm;Zirconium(IV) oxide, 20% in H2O, colloidal dispersion;Zirconium(IV) oxide, Puratronic(R), 99.978% (metals basis) | | CAS: | 1314-23-4 | | MF: | O2Zr | | MW: | 123.22 | | EINECS: | 215-227-2 | | Product Categories: | metal oxide;40: Zr;Biocompatible Ceramics;and Other Ceramics;Biomaterials;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Inorganics;Pyridines;construction;Metal and Ceramic Science;Nanoparticles: Oxides;Nanopowders and Nanoparticle Dispersions;Nitrides;Zirconium;Biocompatible CeramicsMaterials Science;Biocompatible/Biodegradable Materials;40: Zr;Catalysis and Inorganic Chemistry;Materials Science;Nanomaterials;Nanoparticles: Oxides, Nitrides, and Other CeramicsChemical Synthesis;Nanopowders and Nanoparticle Dispersions;Oxides;ZirconiumMetal and Ceramic Science;ZirconiumNanomaterials;1314-23-4 | | Mol File: | 1314-23-4.mol | 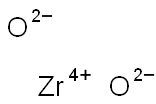 |
| | Zirconium dioxide Chemical Properties |
| Melting point | 2700 °C(lit.) | | Boiling point | 5000 °C(lit.) | | density | 5.89 g/mL at 25 °C(lit.) | | Fp | 5000°C | | solubility | insoluble | | form | powder | | color | White | | Specific Gravity | 5.89 | | PH | 4-5 | | Resistivity | 2.3 × 10*10 (ρ/μΩ.cm) | | Water Solubility | insoluble | | Crystal Structure | Monoclinic; Tetragonal; Cubic | | crystal system | Monoclinic | | Merck | 14,10180 | | Space group | P21/c | | Lattice constant | | a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.51505 | 0.52031 | 0.53154 | 90 | 99.194 | 90 | 0.1406 |
| | Dielectric constant | 12.5(0.0℃) | | Exposure limits | ACGIH: TWA 5 mg/m3; STEL 10 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 | | Stability: | Stable. | | InChIKey | RVTZCBVAJQQJTK-UHFFFAOYSA-N | | CAS DataBase Reference | 1314-23-4(CAS DataBase Reference) | | NIST Chemistry Reference | Zirconium dioxide(1314-23-4) | | EPA Substance Registry System | Zirconium oxide (1314-23-4) |
| | Zirconium dioxide Usage And Synthesis |
| Oral Biomaterials | Crystalline zirconium dioxide (zirconium oxide), ZrO2, called zirconia (not to be confused with zircon, which is a mineral, and Zirkon™, which is a product in the market) is manufactured for use as a white pigment from minerals by conversion to Zr(SO4)2, followed by hydrolysis. ZrO2 is used also as a refractory material (crucibles, furnace lining), and it is insoluble in water, only slightly soluble in HCl and HNO3, and, however, slowly soluble in HF upon heating with 66% H2SO4.

Zirconia is considered one of the best currently known biocompatible ceramic materials along with the metallic titanium.
Zirconium dioxide, or zirconia, ZrO2, is the word in presentday dentistry. We may say that zirconia is a material of choice in contemporary restorative dentistry for several reasons. Moreover, restorative dentistry is about adhesion promotion and about durable bonding of restorations. Zirconia has found wide applications in dental restorations, such as bridges, crowns, dental implant abutments, and full dental implant systems.
Zirconia caught attraction due its superior mechanical properties as superior flexure strength (which is 1200 MPa compared to 1000 MPa for steel), high fracture toughness, high hardness, excellent fatigue, and damage resistance. The material is resistant to chemical attacks and does not react easily with strong acids, alkalis, or other corrosive material. Regarding its physical properties, ZrO2 is a white and opaque material that does not dissolve or react with water and other solvents. It is an excellent thermal and chemical insulator and is used in fuel cells.
| | Uses | Zirconium dioxide (ZrO2) as an abrasive is used to make grinding wheels and special sandpaper. It is also used in ceramic glazes, in enamels, and for lining furnaces and hightemperature molds. It resists corrosion at high temperatures, making it ideal for crucibles and other types of laboratory ware. ZrO2 is used as a "getter" to remove the last trace of air when producing vacuum tubes.
| | Chemical Properties | Heavy, white, amorphous powder. Mohs hardness 6.5, refr index 2.2. Insoluble in water and most acids or alkalies at room temperature; soluble in nitric acid and hot concentrated hydrochloric, hydrofluoric, and sulfuric acids. Most heat resistant of commercial refractories; dielectric. | | Chemical Properties | Zirconium dioxide is a white, amorphous powder, insoluble
in water but slightly soluble in acid. | | Physical properties | Monoclinic zirconia
(baddeleyite structure) stable
below 1197°C, tetragonal
zirconia (rutile structure) stable
between 1197 and 2300°C, cubic
zirconia (fluorine structure)
stable above 2300°C or at lower
temperature if stabilized by
addition of magnesia, calcia or
yttria. Maximum service
temperature 2400°C. Zirconia
starts to act as an oxygen anion
conductor at 1200°C. Highly corrosion resistant to molten
metals such as Bi, Hf, Ir, Pt, Fe,
Ni, Mo, Pu, and V. Strongly
attacked by liquid metals Be, Li,
Na, K, Si, Ti, Zr, and Nb.
Insoluble in water, but slowly
soluble in HCl and HNO3; soluble in boiling concentrated
H2SO4 and alkali hydroxides but readily attacked by HF. | | Physical properties | White, heavy, amorphous powder or monoclinic crystals; refractive index 2.13; density 5.68 g/cm3; Mohs hardness 6.5; transforms to tetragonal structure above 1,100°C and cubic form above 1,900°C; melts at 2,710°C and vaporizes at about 4,300°C; insoluble in water; soluble in hydrofluoric acid and hot sulfuric, nitric and hydrochloric acids. | | Uses | Zirconium oxide (ZrO2) is the most common compound of zirconium found in nature. It
has many uses, including the production of heat-resistant fabrics and high-temperature electrodes
and tools, as well as in the treatment of skin diseases. The mineral baddeleyite (known
as zirconia or ZrO2) is the natural form of zirconium oxide and is used to produce metallic
zirconium by the use of the Kroll process. The Kroll process is used to produce titanium metal
as well as zirconium. The metals, in the form of metallic tetrachlorides, are reduced with magnesium
metal and then heated to “red-hot” under normal pressure in the presence of a blanket
of inert gas such as helium or argon. | | Uses | Instead of lime for the oxyhydrogen light; with earths of the yttrium group in incandescent lighting (Nernst lamps); as pigment, abrasive; manufacture of enamels, white glass, refractory crucibles, and furnace linings. | | Uses | Lanthanum-modified lead zirconate titanate (PLZT) fibers with a diameter of around 300 microns were produced by a thermoplastic processing method. The main use of zirconia is in the production of ceramics with other uses including as a protective coating on particles of titanium dioxide pigments, as a refractory material, in insulation, abrasives and enamels. Stabilized zirconia is used in oxygen sensors and fuel cell membranes because it has the ability to allow oxygen ions to move freely through the crystal structure at high temperatures. This high ionic conductivity (and a low electronic conductivity) makes it one of the most useful electroceramics.Zirconium dioxide is also used as the solid electrolyte in electrochromic devices. Zirconia is a precursor to the electroceramic lead zirconate titanate (PZT), which is a high-K dielectric, which is found in myriad components. | | Uses | Zirconium oxide occurs in nature as the mineral baddeleyite. The oxide has many industrial applications. It is used as a refractory material. It is used in making highly reflective glazes for ceramics, glasses, linings of metallurgical furnaces, crucibles, and laboratory equipment. The oxide is used to produce oxyhydrogen and incandescent lights. Other uses are in producing piezoelectric crystals, heat-resistant fibers, and high-frequency induction coils. The hydrous oxide is used in treating dermatitis resulting from poison ivy. | | General Description | Zirconium(IV) oxide (ZrO2) which is also known as zirconia is a ceramic nanoparticle that can be used as a nano-filler. It can be incorporated in a variety of polymer and metal composites to improve the thermo-mechanical properties of the base material. | | Flammability and Explosibility | Non flammable | | Industrial uses | There are several types of zirconia: a pure(monoclinic) oxide and a stabilized (cubic)form, and a number of variations such asyttria- and magnesia-stabilized zirconia andnuclear grades. Stabilized zirconia has a highmelting point, about 2760°C, low thermal conductivity,and is generally unaffected by oxidizingand reducing atmospheres and mostchemicals. Yttria- and magnesia-stabilized zirconiasare widely used for equipment and vesselsin contact with liquid metals. Monoclinicnuclear zirconia is used for nuclear fuel elements,reactor hardware, and related applicationswhere high purity (99.7%) is needed.Zirconia has the distinction of being an electricalinsulator at low temperatures, graduallybecoming a conductor as temperaturesincrease. | | Carcinogenicity | To simulate the chronic alpha radiation of Thorotrast, the
liver of female Wistar rats was exposed to fractionated
neutron irradiation at 14-day intervals (0.2Gy per fraction)
over 2 years to a total dose of 10.0Gy. Before the start of
irradiation, half of the animals received 120 mL of nonradioactive
Zirconotrast (ZrO2), which is comparable to
Thorotrast in all other physical and chemical properties.
The first liver tumor was detected 1 year after the beginning
of irradiation. At the end of the life span study, the
incidence of irradiated animals with liver tumors was about
40%. In the animals treated additionally with ZrO2, the
incidence, time of onset, and overall number of liver
tumors were nearly equal, indicating that the fractionated
neutron irradiation was the exclusive cause of tumor
development. The lifelong-deposited ZrO2 colloid had
no influence on tumor induction or development. Histological
types of benign and malignant liver tumors seen in
this study were the same as those seen in animals treated
with Thorotrast. |
| | Zirconium dioxide Preparation Products And Raw materials |
|