|
|
| | (αS)-α-Isopropylbenzeneacetic acid Basic information |
| Product Name: | (αS)-α-Isopropylbenzeneacetic acid | | Synonyms: | (2S)-2-Phenyl-3-methylbutanoic acid;(2S)-3-Methyl-2-phenylbutyric acid;(S)-2-Phenyl-3-methylbutanoic acid;(αS)-α-Isopropylbenzeneacetic acid;[S,(+)]-3-Methyl-2-phenylbutyric acid;Benzeneacetic acid, .alpha.-(1-methylethyl)-, (.alpha.S)-;(S)-3-Methyl-2-phenylbutanoic acid;(alphaS)-alpha-(1-Methylethyl)benzeneacetic acid | | CAS: | 13490-69-2 | | MF: | C11H14O2 | | MW: | 178.23 | | EINECS: | | | Product Categories: | | | Mol File: | 13490-69-2.mol | 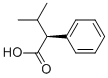 |
| | (αS)-α-Isopropylbenzeneacetic acid Chemical Properties |
| Melting point | 86-88℃ | | Boiling point | 105 °C(Press: 0.1 Torr) | | density | 1.063 | | storage temp. | Sealed in dry,Room Temperature | | pka | 4.26±0.10(Predicted) | | Appearance | White to off-white Solid |
| | (αS)-α-Isopropylbenzeneacetic acid Usage And Synthesis |
| Synthesis | General procedure for the synthesis of (S)-3-methyl-2-phenylbutyric acid from 3-methyl-2-phenylbutyric acid: 2-isopropylphenylacetic acid (8.91 g), (+)-α-phenylethylamine (6.45 mL), and 63% aqueous ethanol solution (193 mL) were mixed, heated and refluxed, then slowly cooled. The precipitated salt was recrystallized three times from 63% aqueous ethanol solution. Subsequently, the salt was treated with 10% sulfuric acid at 0 °C, and the organic layer was extracted with ethyl acetate, dried and concentrated to give 3.52 g (39% yield) of (R)-(-)-2-isopropylphenylacetic acid with a specific optical rotation [α]25D of -58.6° (CHCl3, c=0.02). | | References | [1] European Journal of Medicinal Chemistry, 2017, vol. 140, p. 42 - 51
[2] Tetrahedron, 1971, vol. 27, p. 1173 - 1184
[3] Chemical Communications, 2006, # 34, p. 3600 - 3602 |
| | (αS)-α-Isopropylbenzeneacetic acid Preparation Products And Raw materials |
|