- Neratinib (HKI-272)
-

- $0.00 / 1g
-
2024-03-12
- CAS:698387-09-6
- Min. Order: 1g
- Purity: 98% HPLC
- Supply Ability: 1KG
- Neratinib
-

- $0.00 / 1kg
-
2023-12-23
- CAS:698387-09-6
- Min. Order: 1kg
- Purity: 99%,single impurity<0.1
- Supply Ability: 1 ton
- Neratinib
-
- $0.00 / 1KG
-
2023-11-27
- CAS:698387-09-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 TONS
Related articles - Neratinib- pharmacology
- Neratinib is an oral pan HER inhibitor that irreversibly inhibits the tyrosine kinase activity of epidermal growth factor rece....
- Dec 13,2019
|
| Product Name: | Neratinib | | Synonyms: | Neratinib(HKI-272);(2E)-N-[4-[[3-chloro-4-(2-pyridinylMethoxy)phenyl]aMino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(diMethylaMino)-2-butenaMide;neratinib;(E)-N-[4-[3-Chloro-4-[(2-pyridinyl)methoxy]anilino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-2-butenamide;N-(4-(3-Chloro-4-(2-pyridinylmethoxy)anilino)-3-cyano-7-ethoxy-6-quinolyl)-4-(dimethylamino)-2-butenamide;Unii-jjh94R3pwb;(2e)-n-(4-((3-chloro-4-((pyridin-2-yl)Methoxy)phenyl)aMino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(diMethylaMino)but-2-enaMide neratinib;HKI-272 Neratinib | | CAS: | 698387-09-6 | | MF: | C30H29ClN6O3 | | MW: | 557.04 | | EINECS: | 811-237-1 | | Product Categories: | Inhibitors;Anti-cancer & immunity;Antineoplastic;API | | Mol File: | 698387-09-6.mol | 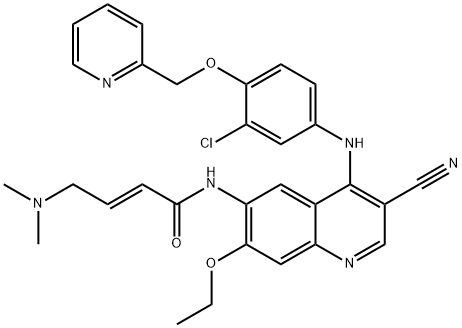 |
| | Neratinib Chemical Properties |
| Melting point | 185-187°C | | Boiling point | 757.0±60.0 °C(Predicted) | | density | 1.33 | | storage temp. | Refrigerator | | solubility | Chloroform (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated) | | form | Off-white solid. | | pka | 12.37±0.43(Predicted) | | color | Pale Yellow to Yellow |
| | Neratinib Usage And Synthesis |
| New anticancer drug | Neratinib developed by US Wyeth company is an irreversible epidermal growth factor receptor(EGFR) inhibitor. It is a multiple target point of small molecule tyrosine kinase inhibitors to HER 2 and HER1 after Lapatinib, and is an irreversible ErbB receptor tyrosine kinase inhibitor. Neratinib could selectively inhibit HER-1 and HER-2 of EGFR family(IC50 was 92 nmol/L and 59 nmol/L, respectively). Clinical research showed that Neratinib exerted significant therapeutic effect on non-small cell lung cancer, colon cancer, and breast cancer.
The phaseⅡclinical trial indicated that Neratinib showed good efficacy and tolerance to HER-2 positive patients with advanced breast cancer who had been received or not Trastuzumab treatment.
The phase Ⅲ breast cancer clinical trial was complete in September 2014. The data indicated that the efficacy of Neratinib was better than Roche's Herceptin in treatment of HER-2 positive early breast cancer.
The above information is edited by the Chemicalbook of Liu Yujie.
| | Genotoxicity | Neratinib and its metabolites were not genotoxic. Administration of neratinib to pregnant rabbits during organogenesis resulted in abortions, embryo-fetal death, and fetal abnormalities at maternal exposures (AUC) approximately 0.2 times exposures in patients at the recommended dose. Oral administration of neratinib to pregnant rats from gestation day 7 until lactation day 20 resulted in effects on long-term memory in male offspring at maternal doses less than the maximum recommended clinical dose on a mg/m2 basis. Neratinib was not carcinogenic in a 26-week carcinogenicity study in rasH2 transgenic mice.
| | Clinical trial | Neratinib was tested in a phase II trial as monotherapy in 2 cohorts of patients with advanced HER2-positive breast cancer those with and those without previous trastuzumab treatment. Sixteen-week progression-free survival (PFS) rates were 59% for patients with previous trastuzumab treatment and 78% for patients with no previous trastuzumab treatment with a median PFS of 22.3 and 39.6 weeks, respectively. Objective response rates were 24% among patients with previous trastuzumab treatment and 56% in the trastuzumab-naive cohort.[4]
| | Synthesis pathways | 3-chloro-4-(pyridin-2-yl-methoxy)-aniline (2) and N-(4-chloro-3-cyano-7-ethoxy-quinolin-6-yl)-acetamide (3) are used as raw material to prepare N-[4-[3-chloro-4-(pyridin-2-yl-methoxy) anilino]-3-cyano-7-ethoxy-quinolin-6-yl] acetamide (4) by nucleophilic substitution. Deprotection of 4 was under the effect of hydrochloric acid, then was precipitated the free base in methanol solution of potassium carbonate to prepare 6-amino-3-cyano-4-[3-chloro-4-(pyridin-2-yl-methoxy) anilino]-7-ethoxy-quinoline (5). Neratinib(1) was obtained by condensation of 5 and acyl chloride which was prepared by trans-4-dimethylamino-crotonic acid hydrochloride (6).
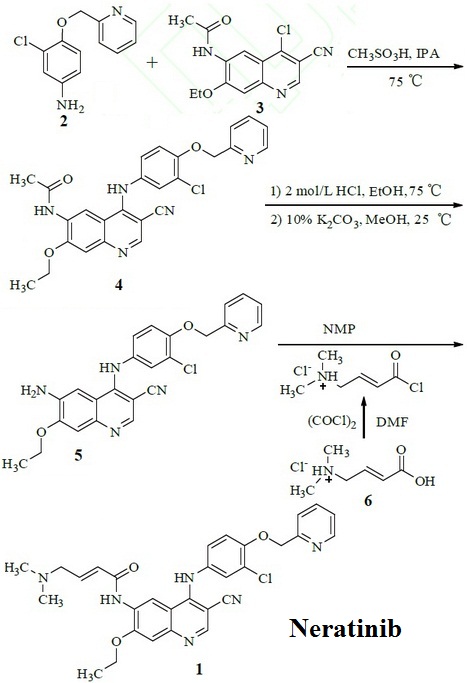
Figure 1 Synthesis pathways of Neratinib
| | Side Effects | Diarrhea, nausea, vomiting, and fatigue.
| | Description | The receptor tyrosine kinase HER2 (ErbB2) is a key component of epidermal growth factor receptor complexes that are known to have central roles in cell proliferation and cancer. Neratinib is an orally active, irreversible inhibitor of the HER2 kinase (IC50 = 59 nM). It also potently inhibits several mutants of HER2 and shows 10-fold or greater selectivity over a wide variety of other kinases. As an irreversible inhibitor, neratinib may circumvent acquired resistance developed against reversible inhibitors, including gefitinib . Neratinib has been evaluated in clinical trials against cancers characterized by HER2 overexpression or HER2 activating mutations. | | Uses | An oral, irreversible dual EGFR/HER2 inhibitor for breast and non-small cell lung cancer. Antitumor agent. | | Uses | Neratinib (HKI-272) is a highly selective HER2 and EGFR inhibitor with IC50 of 59 nM and 92 nM, respectively. | | Definition | ChEBI: A quinoline compound having a cyano group at the 3-position, a 3-chloro-4-(2-pyridylmethoxy)anilino group at the 4-position, a 4-dimethylamino-trans-but-2-enamido group at the 6-position, and an ethoxy group at the 7-position. | | target | HER2 | | storage | Store at -20°C |
| | Neratinib Preparation Products And Raw materials |
|