| Company Name: |
Sadasivarao g
|
| Tel: |
+91 9515157878 |
| Email: |
sada@octanexlabs.com |
| Products Intro: |
Product Name:Tert-butyl(3S)-3-methylsulfonyloxypiperidine-1-carboxylate
CAS:940890-90-4
Purity:99% Package:1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
Carbott PharmTech Inc.
|
| Tel: |
0535-6385396 |
| Email: |
info@carbottpharm.com |
| Products Intro: |
Product Name:Tert-butyl (3S)-3-methylsulfonyloxypiperidine-1-carboxylate
CAS:940890-90-4
Purity:97% Package:10g;100g;1kg
|
|
| | (S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE Basic information |
| Product Name: | (S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE | | Synonyms: | (S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE;1-Piperidinecarboxylic acid, 3-[(Methylsulfonyl)oxy]-, 1,1-diMethylethyl ester, (3S)-;1Piperidinecarboxylic acid, 3[(methylsulfonyl)oxy], 1,1dimeth;2-Methyl-2-propanyl (3S)-3-[(methylsulfonyl)oxy]-1-piperidinecarboxylate
(S)tertButyl 3(methylsulfonyloxy)piperidine1carboxylate;(S)-1-Boc-3-mesyloxyl-piperidine;Tert-butyl (3S)-3-methylsulfonyloxypiperidine-1-carboxylate;(S)-1-BOC-3-(Methylsulfonyloxy)piperidine;(methylsulfonyloxy) | | CAS: | 940890-90-4 | | MF: | C11H21NO5S | | MW: | 279.35 | | EINECS: | | | Product Categories: | | | Mol File: | 940890-90-4.mol | 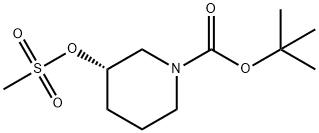 |
| | (S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE Chemical Properties |
| storage temp. | 2-8°C | | Appearance | White to off-white Solid |
| | (S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE Usage And Synthesis |
| Uses | tert-Butyl (3S)-3-[(Methylsulfonyl)oxy]piperidine-1-carboxylate is used as a reactant in the synthesis of non-nucleoside MtbTMPK inhibitors. | | Synthesis | Under nitrogen protection, (S)-1-Boc-3-hydroxypiperidine (10.0 g, 49.7 mmol) and triethylamine (7.82 mL, 54.7 mmol) were dissolved in dichloromethane (60 mL) and cooled to 0 °C. Subsequently, methanesulfonyl chloride (4.23 mL, 54.7 mmol) was slowly added dropwise and the reaction mixture was stirred at this temperature for 1 hour. Upon completion of the reaction, the reaction solution was diluted with deionized water (60 mL) and filtered through a hydrophobic glass stock. The organic layer was concentrated under reduced pressure to afford tert-butyl (3S)-3-(methylsulfonyloxy)piperidine-1-carboxylate (13.9 g, 49.7 mmol, 100% yield) as a colorless solid. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ (ppm) 4.76-4.71 (m, 1H, CH), 3.69-3.66 (m, 1H), 3.65-3.60 (m, 1H), 3.49-3.43 (m, 1H), 3.37-3.31 (m, 1H), 3.07 (s, 3H, CH3) , 2.02-1.96 (m, 1H), 1.94-1.91 (m, 1H), 1.87-1.80 (m, 1H), 1.59-1.53 (m, 1H), 1.48-1.45 (m, 9H). | | References | [1] Patent: WO2014/188173, 2014, A1. Location in patent: Paragraph 00224
[2] Patent: CN105399756, 2016, A. Location in patent: Paragraph 0152; 0167-0168
[3] MedChemComm, 2014, vol. 5, # 12, p. 1879 - 1886
[4] Patent: WO2017/134684, 2017, A2. Location in patent: Page/Page column 12
[5] Patent: CN103864673, 2016, B. Location in patent: Paragraph 0084; 0085 |
| | (S)-1-(TERT-BUTOXYCARBONYL)PIPERIDIN-3-YL METHANESULFONATE Preparation Products And Raw materials |
|