- Alliin
-

- $5.00 / 25kg
-
2024-04-24
- CAS:556-27-4
- Min. Order: 1kg
- Purity: 99.92%
- Supply Ability: 50000tons
|
| Product Name: | Alliin | | Synonyms: | Allylsulphinyl-L-alanine;(S)-3-(ALLYLSULFINYL)-L-ALANINE;(S)-S-ALLYL-L-CYSTEINE SULFOXIDE;(S)-3-(allylsulphinyl)-L-alanine;S-Allyl-L-cysteine Sulfoxide;ALLIIN(P);ALLIIN hplc;(2R)-2-Amino-3-[(S)-prop-2-enylsulfinyl]propanoic acid | | CAS: | 556-27-4 | | MF: | C6H11NO3S | | MW: | 177.22 | | EINECS: | 209-118-9 | | Product Categories: | Sulphur Derivatives;Miscellaneous Natural Products | | Mol File: | 556-27-4.mol | 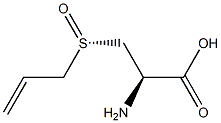 |
| | Alliin Chemical Properties |
| Melting point | 164-166° (effervescence) | | alpha | D20 +63.5° (c = 2) | | Boiling point | 416.1±45.0 °C(Predicted) | | density | 1.205 (estimate) | | refractive index | 1.5500 (estimate) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | | form | solid | | pka | 1.88±0.10(Predicted) | | color | White to off-white | | Merck | 13,259 | | InChI | InChI=1/C6H11NO3S/c1-2-3-11(10)4-5(7)6(8)9/h2,5H,1,3-4,7H2,(H,8,9)/t5-,11-/s3 | | InChIKey | XUHLIQGRKRUKPH-DYEAUMGKSA-N | | SMILES | C(O)(=O)[C@H](C[S@](CC=C)=O)N |&1:3,5,r| | | LogP | -0.478 (est) | | CAS DataBase Reference | 556-27-4(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26 | | WGK Germany | 3 | | F | 8-10 | | HS Code | 29309090 |
| | Alliin Usage And Synthesis |
| Description | Alliin (S-allyl cysteine sulfoxide) is an organic sulfur-containing product derived from the amino acid cysteine. It is the precursor of thiosulfinates, which are responsible for the characteristic pungent flavours of Allium species. It is an L-alanine derivative in which one of the methyl hydrogens of L-alanine has been replaced by an (S)-allyl sulfinyl group. It has a role as a plant metabolite, an antioxidant, a cardioprotective agent, a neuroprotective agent and an antimicrobial agent. It is a non-proteinogenic alpha-amino acid, an L-cysteine derivative, an L-alanine derivative, a sulfoxide and an olefinic compound. It is a tautomer of an alliin zwitterion. | | Occurrence |
Alliin is a sulfoxide that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Garlic powder is not a source of alliin, nor is fresh garlic upon maceration since the enzymatic conversion to allicin takes place in the order of seconds. Alliin was the first natural product with both carbon- and sulfur-centered stereochemistry[1].
| | Uses | antibacterial, antioxidant | | Definition | ChEBI: An L-alanine derivative in which one of the methyl hydrogens of L-alanine has been replaced by an (S)-allylsulfinyl group. | | benefits | Different from allicin, which is quickly metabolized into diallyl disulfide and allyl mercaptan, alliin with bioavailability of 16.5% has nearly no first liver pass effect except that some are converted to diallyl disulfide. An extensive literature review discloses the nutraceutical and medicinal potential of alliin in several aspects. For example, alliin decreased fasting glucose levels and increased insulin levels to the same extent as glibenclamide, glyclazide or insulin in diabetic rats. Weeks’ administration of alliin modified the redox environment by decreasing reactive substances in organs and increasing the catalase and superoxide dismutase activities in nicotine-fed rats. Another study showed that alliin markedly depressed the increase of plasma and liver cholesterol levels in rats fed a hypercholesterolemic diet containing 10% hydrogenated coconut oil, 1% cholesterol and 0.2% cholic acid. Alliin was also found to possess anti-inflammatory activities for bowel diseases. These symptoms like diabetes, high radicals, hypercholesterolemia and inflammation are all associated with obesity and/or metabolic diseases. Supportively, a meta-analysis suggests that the single component intake of garlic may be effective for lowering plasma glucose concentration and body weight[1]. | | target | PPAR | TNF-α | IL Receptor | NF-kB | SOD | NADPH-oxidase | ROS | | References | [1] Baiqiang Zhai. “Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice.” Scientific Reports (2018): 3527.
|
| | Alliin Preparation Products And Raw materials |
|