(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol: properties, synthesis and safety
Dec 13,2023
General Description
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol is a chiral alcohol derivative with unique properties, including its clear and colorless appearance and moderate stability. It is sparingly soluble in water but dissolves readily in organic solvents. The compound has various applications in pharmaceutical and chemical research, particularly as a chiral intermediate for crizotinib, a targeted therapy drug used in cancer treatment. The synthesis of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol involves the use of alcohol dehydrogenases and glucose dehydrogenase, resulting in high enantiomeric excess values. However, proper safety measures are necessary when handling this compound due to its potential harmful effects on human health and the environment. Overall, (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol shows promise for cost-effective and sustainable large-scale manufacturing of crizotinib and other novel drug molecules.

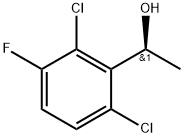
Figure 1. (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
Properties
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol is a chemical compound with unique properties. It is an alcohol derivative that consists of a chiral center, indicated by the "S" in its name. This chiral center gives rise to two enantiomers, namely the (S)- and (R)-forms. However, for the purpose of this description, we will focus solely on the (S)-enantiomer. This compound exhibits a molecular formula of C8H7Cl2FOH and a molecular weight of approximately 209.04 g/mol. It possesses a clear and colorless appearance, making it visually distinctive. (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol is sparingly soluble in water but dissolves readily in common organic solvents such as ethanol and acetone. The compound's physical properties include a boiling point of around 261.3°C. It is important to note that these values may vary slightly depending on experimental conditions. In terms of reactivity, (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol demonstrates moderate stability under normal conditions. However, it can undergo various chemical transformations through reactions such as esterification, etherification, and oxidation, enabling its applicability in synthetic organic chemistry. Overall, the distinctive properties of (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol make it a valuable compound in pharmaceutical and chemical research, providing opportunities for the development of novel drug molecules and synthetic intermediates. 1
Synthesis
(S)-1-(2,6-dichloro-3-fluorophenyl)ethanol, a chiral intermediate of crizotinib, has various applications in the pharmaceutical industry. Crizotinib is a targeted therapy drug used in the treatment of non-small cell lung cancer (NSCLC) and certain types of advanced-stage tumors. The synthesis of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol involves the use of alcohol dehydrogenases from Lactobacillus kefir (ADH-LK) with a tetrad mutant (ADH-LKM) and glucose dehydrogenase (GDH). Through heterologous expression in recombinant Escherichia coli, ADH-LKM and GDH were successfully produced. The enzyme system efficiently converts 1-(2,6-dichloro-3-fluorophenyl)ethanone, a precursor compound, into the desired chiral alcohol. Under optimal conditions (30 °C, pH 7.0), a high enantiomeric excess value of 99.9% was achieved after 12 hours, with complete conversion of the substrate at a concentration of 150 g/L. The successful production of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol demonstrates the potential for large-scale and environmentally friendly manufacturing of this key chiral intermediate. This advancement in synthesis technology opens up opportunities for cost-effective and sustainable production of crizotinib, contributing to the availability and affordability of this crucial medication for cancer patients. 2
Safety
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol possesses certain safety considerations that need to be taken into account. It is important to handle this compound with caution to ensure personal and environmental safety. Direct contact with the skin should be avoided as it can be harmful and may cause irritation. Individuals with sensitive skin may experience allergic reactions upon exposure. Protective measures such as gloves and appropriate clothing should be worn when handling this compound. The compound is also known to cause serious damage and irritation to the eyes. Eye protection, such as goggles, should be used to prevent any accidental splashes or exposure. In terms of respiratory safety, (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol may cause respiratory irritation. Adequate ventilation should be ensured in areas where this compound is being used or processed. Furthermore, it is important to note that (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol can have harmful effects on aquatic life with long-lasting consequences. Therefore, proper measures should be taken to prevent its release into water bodies and to avoid contamination. Overall, strict adherence to safety protocols, including proper protective equipment and handling procedures, is essential when working with (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol to mitigate any potential risks to human health and the environment. 3
Reference
1. PubChem. COMPOUND SUMMARY: (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol. National Library of Medicine PubChem CID: 11557536
2. Zong C, Zhang X, Yang F, Zhou Y, Chen N, Yang Z, Ding G, Yu F, Tang Y. Biotransformation of a crizotinib intermediate using a mutant alcohol dehydrogenase of Lactobacillus kefir coupled with glucose dehydrogenase. Prep Biochem Biotechnol. 2019;49(6):578-583.
3. (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol. European Chemicals Agency, EC / List no. 700-699-9.
- Related articles
- Related Qustion
- What is (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol? Feb 24, 2020
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol is a small molecule kinase inhibitor for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) positive for anaplastic lymphohematokinase (ALK).
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDoramectin is a traditional anthelmintic veterinary drug that has been used to treat helminthic infections, not only to relieve clinical signs but also to cure oesophageal nodules, and is the drug of choice in many endemic countries.....
Dec 13,2023Drugs(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
877397-65-4You may like
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol manufacturers
- (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
-
- $0.00 / 25kg
- 2025-12-15
- CAS:877397-65-4
- Min. Order: 1kg
- Purity: >98% by HPLC
- Supply Ability: 10kg/month
- 5-Formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid
-

- $0.00 / 10g
- 2025-12-12
- CAS:253870-02-9
- Min. Order: 10g
- Purity: 99%+
- Supply Ability: 10000KG
- (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
-

- $10.00 / 1KG
- 2025-12-11
- CAS:877397-65-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt