1,1,1,3,3,3-Hexafluoro-2-propanol: Application, synthesis, toxicity and environmental hazard
May 17,2023
General description
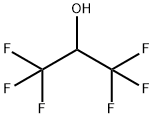
1,1,1,3,3,3-Hexafluoro-2-propanol, commonly abbreviated HFIP, is the organic compound finds use as solvent and synthetic intermediate. As a solvent 1,1,1,3,3,3-Hexafluoro-2-propanol is polar and exhibits strong hydrogen bonding properties enabling it to dissolve substances that serve as hydrogen-bond acceptors, such as amides and ethers. It is classified as a hard Lewis acid and its acceptor properties are discussed in the ECW model. Its relative acceptor strength toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots. 1,1,1,3,3,3-Hexafluoro-2-propanol is transparent to UV light with high density, low viscosity and low refractive index. Although 1,1,1,3,3,3-Hexafluoro-2-propanol has a certain viscosity, its boiling point is very low and usually requires analysis at 40 °C to reduce the back pressure of the chromatographic column. It is also a highly polar solvent, which can cause the calibration curve obtained by mixed column bed chromatography to become non-linear. Its appearance is as follows:

Figure 1 Appearance of 1,1,1,3,3,3-Hexafluoro-2-propanol.
Application
1,1,1,3,3,3-Hexafluoro-2-propanol is a speciality solvent for some polar polymers and organic synthesis.[1] It is especially effective for solubilizing a wide range of polymers, including those that are not soluble in the most common organic solvents, such as: polyamides, polyacrylonitriles, polyacetals, polyesters (e.g. polyglycolide), and polyketones. 1,1,1,3,3,3-Hexafluoro-2-propanol has also found use in biochemistry to solubilize peptides and to monomerize β-sheet protein aggregates. Because of its acidity (pKa = 9.3), it can be used as acid in volatile buffers for ion pair HPLC - mass spectrometry of nucleic acids.[2] 1,1,1,3,3,3-Hexafluoro-2-propanol is both the precursor and the chief metabolite of the inhalation anesthetic sevoflurane. In addition, 1,1,1,3,3,3-Hexafluoro-2-propanol is an important fluorinated fine chemical that can be used to prepare various fluorinated chemicals such as fluorinated surfactants, fluorinated emulsifiers, and fluorinated pharmaceuticals. It can improve the efficiency of rhodium (I) - catalyzed intramolecular addition reaction reaction of alkynyldiene bound by ether and cycloaddition reaction of alkynylvinylcyclopropane.
Synthesis
1,1,1,3,3,3-Hexafluoro-2-propanol can be synthesized by the following steps: A suspension of 63g (642 mmol, 2.5 equiva-lents) of potassium acetate in 250 ml of dimethyl forma-mide is given into a reaction vessel. Under nitrogen, 257 mmol of CF3-CHCCF3 are introduced. The reaction mixture is stirred overnight at room temperature and mixed with water and a saturated aqueous NaCl solution. After drying over Na2SO4, the supernatant liquid is filtrated off, concentrated at reduced pressure, dissolved in ethanol and mixed with approximately 3 ml of concentrated H2SO4. After stirring overnight, the reaction mixture is concentrated under vacuum, and mixed with water and ethyl acetate. The organic phase is washed with water until the pH is neutral, and then dried over Na2SO4. After 7 hours, the heating and stirring was stopped and the reaction solution was analyzed by GC and NMR. Hexafluoroisopropanol is disted out of the residue. Final product. boiling point 58.2°C. Selectivity of 1,1,1,3,3,3-Hexafluoro-2-propanol by GC analysis was 99.95% [3].
Toxicity
1,1,1,3,3,3-Hexafluoro-2-propanol is a volatile, corrosive liquid that can cause severe burns and respiratory problems. With prolonged or repeated exposure, it is likely to impair fertility as well as harm the unborn child.
Environmental hazard
The release of 1,1,1,3,3,3-Hexafluoro-2-propanol into the environment must be avoided. It belongs to the per- and polyfluoroalkyl substances. These so-called PFAS, also known as forever chemicals, are considered non-degradable substances in nature due to their fluorination. They are therefore classified as persistent organic pollutants. Due to its good solubility in water, 1,1,1,3,3,3-Hexafluoro-2-propanol is also highly mobile. Thus, it counts as a vPvM/PMT substance (very persistent, mobile substances that may have toxic potential).[4] As a volatile and fluorinated substance, 1,1,1,3,3,3-Hexafluoro-2-propanol increases global warming. It has a global warming potential (GWP) of 195, making it 195 times more harmful to the atmosphere than CO2.
References
[1]Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B. (2004). "Fluorinated Alcohols: A New Medium for Selective and Clean Reaction". Synlett (1): 18–29.
[2]Apffel, A.; Chakel, J.A.; Fischer, S.; Lichtenwalter, K.; Hancock, W.S. (1997). "Analysis of oligonucleotides by HPLC-electrospray ionization mass spectrometry". Anal. Chem. 69: 1320–1325.
[3]Braun et al. Manufacture of hexafluoroisopropanol. European Patent Organization. Patent Number: EP2626341.
[4]Arp, Hans Peter (November 2019). REACH: Improvement of guidance and methods for the identification and assessment of PMT/vPvM substances (PDF). Umweltbundesamt. Retrieved 7 January 2021.
- Related articles
- Related Qustion
- The wide range of applications: 1,1,1,3,3,3-Hexafluoro-2-propanol Apr 26, 2024
Hexafluoro-2-propanol (HFIP; 1,1,1,3,3,3-Hexafluoro-2-propanol) is a low-boiling solvent used in a variety of chemical applications.
- What is 1,1,1,3,3,3-Hexafluoro-2-propanol? Dec 30, 2019
1,1,1,3,3,3-Hexafluoro-2-propanol also known as hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound with the formula (CF3)2CHOH. This fluoroalcohol finds use as solvent and synthetic intermediate.
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a barium salt and a metal sulfate.....
May 17,2023Inorganic salts2,2'-Azobis(2-methylpropionitrile) is used as an initiator in polymer radical polymerization because its molecules can easily undergo split reactions and form molecules with high activation energy.....
May 17,2023Organic Synthesis Intermediate1,1,1,3,3,3-Hexafluoro-2-propanol
920-66-1You may like
1,1,1,3,3,3-Hexafluoro-2-propanol manufacturers
- 1,1,1,3,3,3-Hexafluoro-2-propanol
-
- 2025-12-14
- CAS:920-66-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Mung bean peptide
-

- $0.00 / 1KG
- 2025-12-13
- CAS:
- Min. Order: 1KG
- Purity: 75% HPLC
- Supply Ability: 1000KG
- 1,1,1,3,3,3-Hexafluoro-2-propanol
-

- $0.00 / 200kg
- 2025-12-12
- CAS:920-66-1
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 500mt